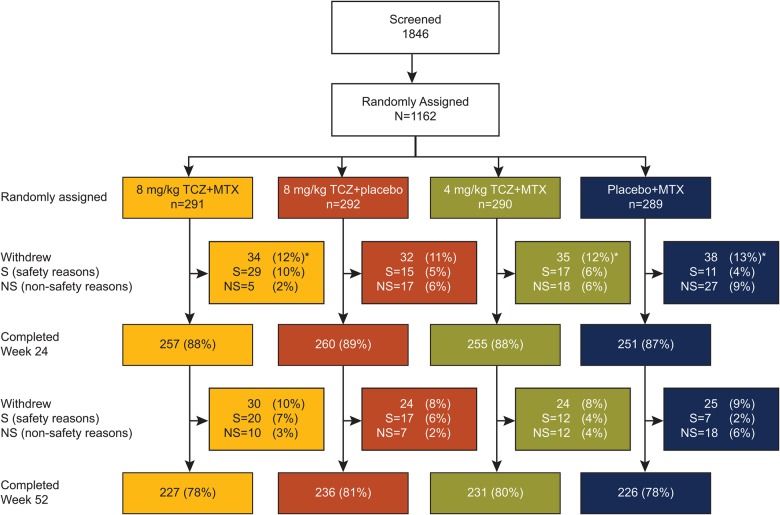
Figure 1.
Patient disposition. *Five patients (two in the placebo+MTX group, two in the 4 mg/kg TCZ+MTX group and one in the 8 mg/kg TCZ+MTX group) did not receive study treatment and were excluded from analysis populations. Withdrawals in the placebo+MTX group were mainly driven by insufficient therapeutic response and refused treatment; withdrawals in the TCZ combination therapy groups were mainly related to safety (primarily hepatic transaminase elevations). Two patients randomly assigned to the placebo+MTX group received TCZ at the baseline visit and were allocated to the 4 mg/kg TCZ+MTX group for safety analysis. The ITT population comprised 1157 patients, and the safety population comprised 1153 patients. ITT, intent-to-treat; MTX, methotrexate; TCZ, tocilizumab.