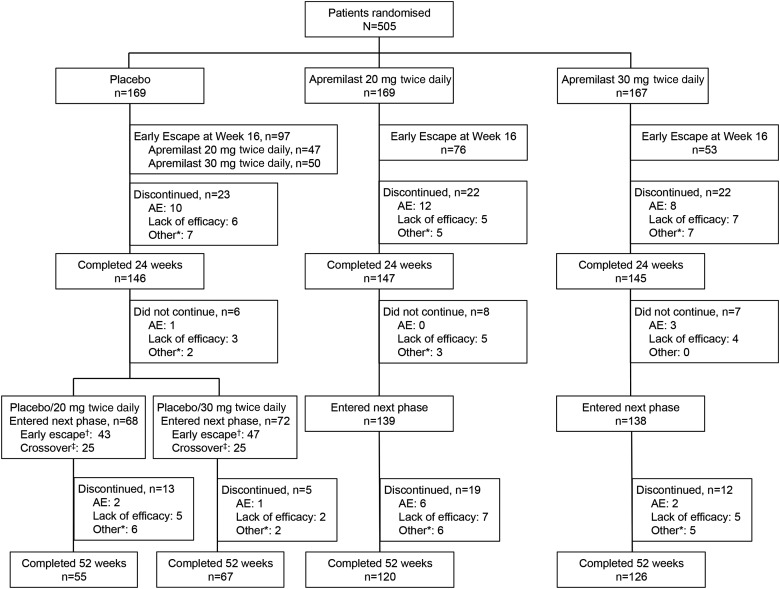
Figure 1.
Patient disposition through week 52. *Includes withdrawal by patient, lost to follow-up, protocol violation, non-compliance, other. †Includes patients initially randomised to placebo, who met early escape criteria and were then randomised to apremilast at week 16. ‡Includes patients initially randomised to placebo who were then randomised to apremilast at week 24 for the active treatment phase (week 24–52). AE, adverse event.