Figure 2.
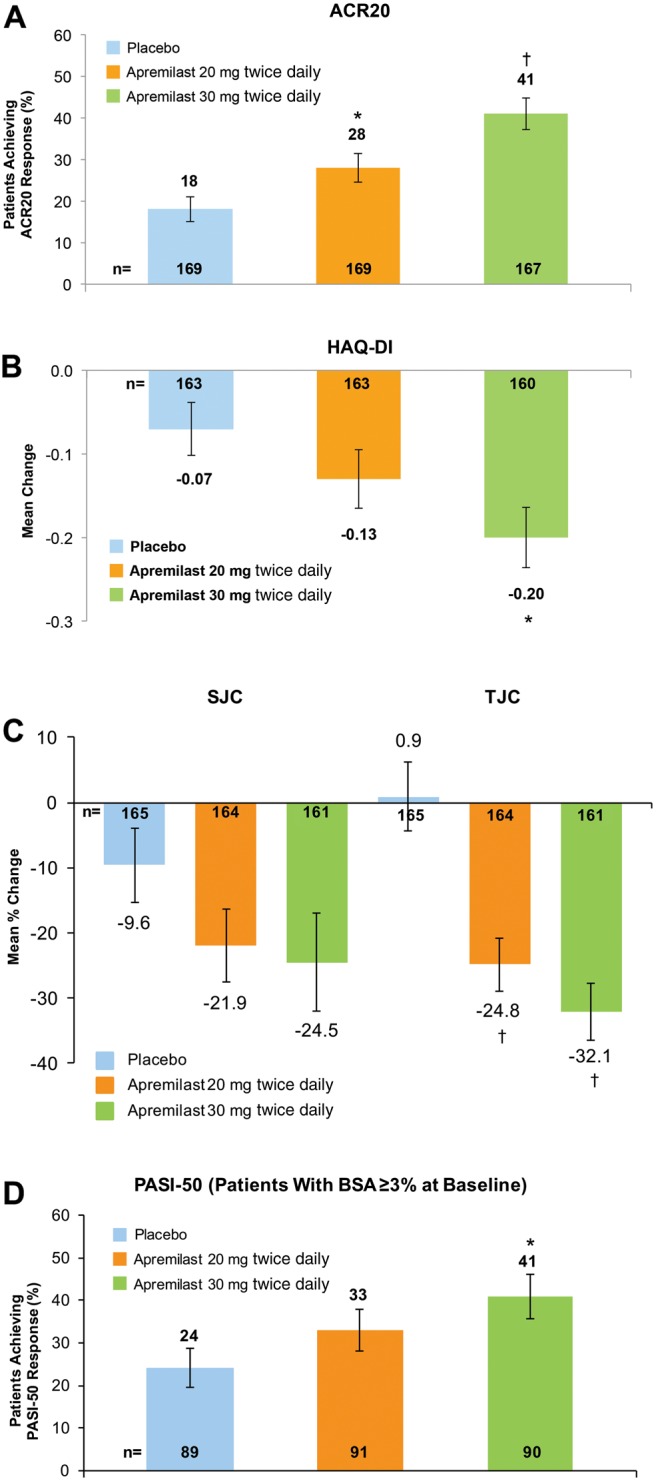
ACR20 (A), HAQ-DI (B), SJC and TJC (C) and PASI-50 (D) at week 16. *p<0.05; †p≤0.0001 versus placebo, based on analysis of covariance model for HAQ-DI, SJC and TJC, and Cochran-Mantel-Haenszel test for ACR20 and PASI-50 for the intent-to-treat population; patients who discontinued or did not have sufficient data were counted as non-responders (ACR20 and PASI-50) or had their last observation was carried forward (HAQ-DI, SJC and TJC). Error bars represent SE. ACR, American College of Rheumatology; BSA, body surface area; HAQ-DI, Health Assessment Questionnaire-Disability Index; PASI-50, 50% reduction from baseline Psoriasis Area and Severity Index; SJC, swollen joint count; TJC, tender joint count.
