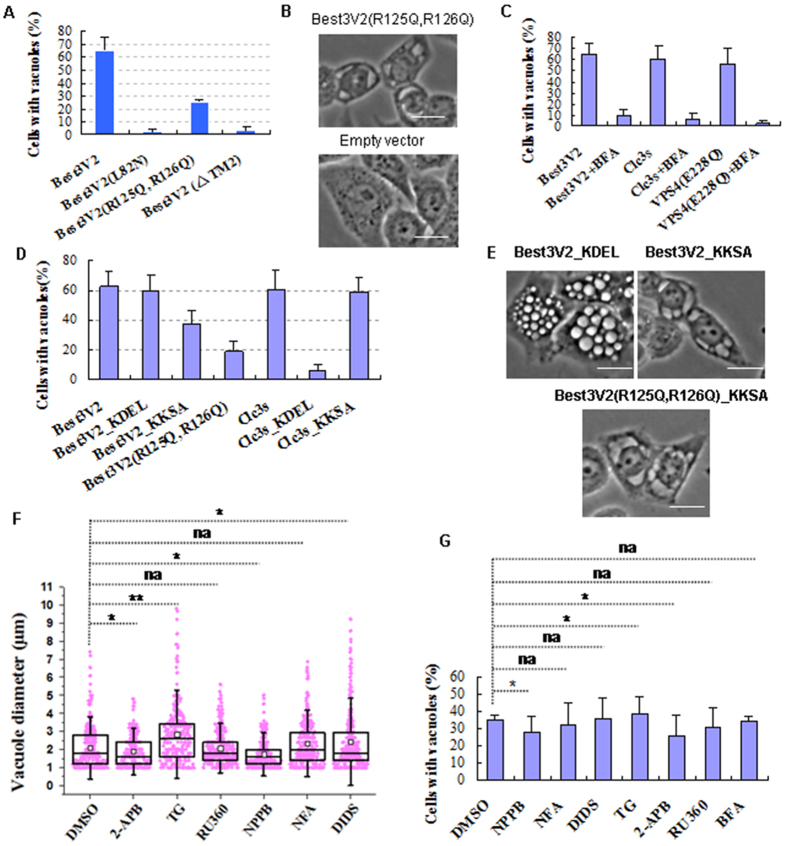
Figure 4. Retention of Best3V2 in the ER also results in ER vacuolization.
(A) Mutation of TM2 (L82N), mutation of 2 amino acids (R125Q and R126Q) in its phospholipid binding domain, or deletion of TM1 and TM2 (natural splice variant V1 of Best3) abolished Vac-V2 formation in HeLa cells. (B) Low-diopter vacuoles induced by Best3V2(R125Q, R126Q) in HeLa cells. Cells that were transfected with the empty vector were used as the control. (C) Bafilomycin A1 (BFA) strongly decreased vacuole formation. The same treatment was also applied to the Clc3s and VPS4(E228Q) as parallel controls. (D) Distinct impact of the ER luminal side retention signal _KDEL and ER cytosolic side retention signal _KKXX (KKSA) on the generation of Vac-V2 or Vac-Clc3s. (E) Morphology of vacuoles induced by Best3V2_KDEL, Best3V2_KKSA or Best3V2(R125Q,R126Q)_KKSA in HeLa cells. (F) The impact of ion channel blockers on Vac-V2 formation. One hundred vacuole-positive HeLa cells were used for counting. *p < 0.05. **p < 0.01. (G) Several channel blockers inhibited Best3V2_KKSA-induced ER vacuole formation in Hela cells. The error bars denote the S.D. values. Scale bar, 20 μm. The experiments were repeated 3 times.