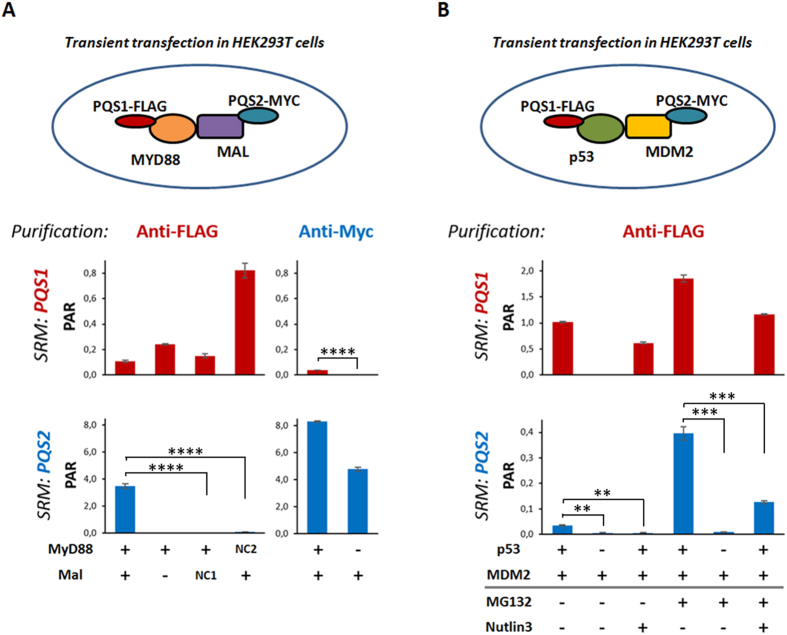
Figure 4. Use of PQS peptides to study binary interactions between tagged proteins upon expression in human cells.
Peak Area Ratios to heavy (PAR) of summed transitions for PQS1 and PQS2 are shown for three experimental setups in HEK293T cells. In each sample, stable isotope labeled peptide were added to a final injected amount of 80 fmol. Each depicted sample was run in technical triplicate, error bars represent mean error values. A representative experiment is shown for 3 independent biological repeats. (A) Interaction between Mal and MyD88 as shown by PQS peptide quantification. Different combinations of FLAG-PQS1-MyD88, Myc-PQS2-Mal and mock interactors (Myc-PQS2-MARK3 (NC1) and FLAG-PQS1-Ras (NC2)) were transfected. After immuno-precipitation using anti-FLAG or anti-Myc followed by on-bead tryptic digest, the levels of PQS1 and PQS2 were quantified by SRM. Independent t-test; all p values <0.0001. (B) PQS peptide quantification for the interaction between p53 and MDM2. Different combinations of FLAG-PQS1-p53 and Myc-PQS2-MDM2 were expressed in human cells. The interaction was monitored in absence or presence of the proteasome inhibitor (MG132) or a small-molecule inhibitor of the p53 - MDM2 interaction (Nutlin3). A significance drop in signal by Nutlin3 addition could be observed both in absence and presence of MG132 (Independent t-test, p values: 0.0012 and 0.0006 in absence and presence of MG132, respectively).