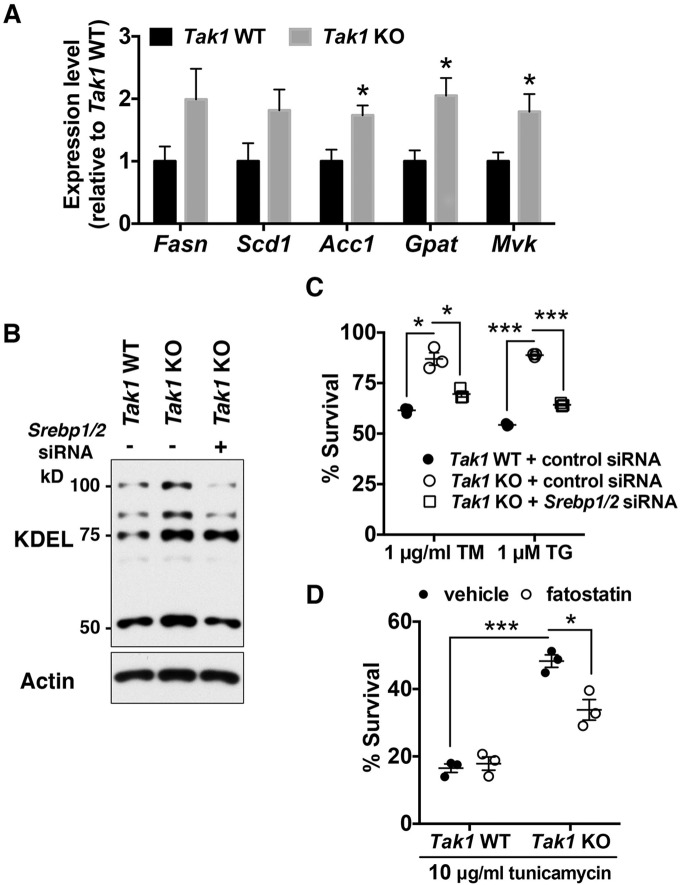
Fig. 3.
Tak1-deficiency enhances ER-stress tolerance through SREBPs. (A) mRNA levels of SREBP-target genes in Tak1 wild-type (Tak1 WT) and Tak1-deficient (Tak1 KO) fibroblasts were analyzed by performing quantitative real-time PCR. All mRNA levels were quantified relative to Gapdh gene expression and presented as the fold change relative to levels in Tak1 WT fibroblasts. Fasn, fatty acid synthase; Scd1, stearyl coenzyme-A desaturase1; Acc1, acetyl-CoA carboxylase1; Gpat, glycerol-3-phosphate acyltransferase; Mvk, mevalonate kinase; n=6 per genotype; mean±s.e.m.; *P<0.05; **P<0.01; ***P<0.001. (B,C) Tak1 WT and Tak1 KO fibroblasts were transfected with non-targeting control siRNA or a mixture of siRNAs against Srebp1 and Srebp2 (Srebp1/2). At 72 h post transfection, cell lysates were analyzed by immunoblotting with anti-KDEL antibody (B). Alternatively, at 72 h post transfection, cells were treated with vehicle alone, 1 μg/ml tunicamycin (TM) or 1 μM thapsigargin (TG) for 18 h. Cell viability was measured by Crystal Violet staining (C). Expression levels of Srebp1 and Srebp2 were effectively downregulated by the transfection of siRNAs (Fig. S2D). n=3 per treatment; mean±s.e.m.; *P<0.05; ***P<0.001. (D) Tak1 WT and Tak1 KO fibroblasts were pre-treated with vehicle alone or 10 μg/ml of fatostatin for 24 h. Cells were then treated with either vehicle or 10 μg/ml tunicamycin for 18 h. Cell viability was measured by Crystal Violet staining. n=3 per treatment; mean±s.e.m.; *P<0.05; ***P<0.001.