Abstract
Introduction
Paediatric central venous access devices (CVADs) are associated with a 25% incidence of failure. Securement and dressing are strategies used to reduce failure and complication; however, innovative technologies have not been evaluated for their effectiveness across device types. The primary aim of this research is to evaluate the feasibility of launching a full-scale randomised controlled efficacy trial across three CVAD types regarding CVAD securement and dressing, using predefined feasibility criteria.
Methods and analysis
Three feasibility randomised, controlled trials are to be undertaken at the Royal Children's Hospital and the Lady Cilento Children's Hospital, Brisbane, Australia. CVAD securement and dressing interventions under examination compare current practice with sutureless securement devices, integrated securement dressings and tissue adhesive. In total, 328 paediatric patients requiring a peripherally inserted central catheter (n=100); non-tunnelled CVAD (n=180) and tunnelled CVAD (n=48) to be inserted will be recruited and randomly allocated to CVAD securement and dressing products. Primary outcomes will be study feasibility measured by eligibility, recruitment, retention, attrition, missing data, parent/staff satisfaction and effect size. CVAD failure and complication (catheter-associated bloodstream infection, local infection, venous thrombosis, occlusion, dislodgement and breakage) will be compared between groups.
Ethics and dissemination
Ethical approval to conduct the research has been obtained. All dissemination will be undertaken using the CONSORT Statement recommendations. Additionally, the results will be sent to the relevant organisations which lead CVAD focused clinical practice guidelines development.
Trial registration numbers
ACTRN12614001327673; ACTRN12615000977572; ACTRN12614000280606.
Keywords: central venous catheter, site care, dressing, evidence-based practice, protocol
Strengths and limitations of this study.
Pilot randomised controlled design to enhance reliability of results using predetermined primary outcomes of feasibility.
Securement and dressing products being trialled are not amenable to blinding of patients, family members, clinical staff or research staff. Radiological and laboratory staff assessing outcomes will be blinded.
Use of computer-generated randomisation and allocation concealment will avoid risk of selection and allocation bias.
Introduction
Central venous access devices (CVADs) are used for monitoring and medication in critically and chronically unwell patients in a variety of inpatient and outpatient settings.1 2 More than 5 million CVADs are used in the USA per year alone.3 Conventionally, non-tunnelled CVADs (nt-CVADs) have been advocated for use when central venous access is required for a short time,4–6 peripherally inserted central catheters (PICCs) for short-to-medium time,4 6 and tunnelled CVADs (t-CVAD) and totally implantable devices for longer time periods.6 7
Children requiring CVADs to facilitate treatment are extremely vulnerable to the risk of adverse events associated with insertion and management.8 9 About 25% of paediatric CVADs fail prior to treatment being complete.10 This includes CVADs becoming partially or wholly dislodged, occlusions, venous thrombosis, fractured catheters, site erosion, severe pain or a bloodstream infection. The consequences of failure include the morbidity and mortality associated with the cause of the complication (eg, catheter-associated bloodstream infection (CABSI); with an attributable mortality as high as 35%),11 12 interruption of medical treatment and the insertion of replacement CVADs, involving the additional risk of procedural complications. Many CVAD complications are preventable with the consistent use of evidence-based CVAD insertion and maintenance practices.6 13 14
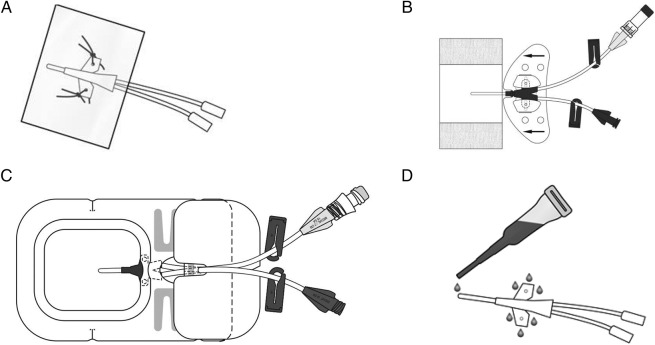
An essential component to prevent postinsertion CVAD complications is the securement and dressing product chosen. To prevent complications, CVADs require (1) insertion site protection from microbial contamination from the surrounding skin and environment; (2) the external portion to be secured to prevent venous dislodgement and (3) securement to prevent micromotion within the vein and at the insertion site.15 Micromotion is believed to irritate the vein wall causing inflammation, thrombosis, occlusion, vessel erosion and encourages the skin bacteria to enter the insertion wound.15–17 Since the 1980s, pervasive practice has been to suture CVADs for securement, with adhesive, polyurethane dressings placed over the sutured site (see figure 1A).18 Transparent polyurethane dressings are claimed to be impermeable to microorganisms but semipermeable to oxygen, carbon dioxide and water vapour.11 15 18
Figure 1.
Illustration of products tested within the CASCADE junior trials: (A) Simple polyurethane and suture; (B) Sutureless securement device with simple polyurethane; (C) Integrated securement dressing product; (D) Tissue adhesive.
Recent evidence supports the introduction of chlorhexidine gluconate-impregnated (CGI) CVAD dressing products within the critical care as a strategy to reduce the incidence of site colonisation and CABSI in non-tunnelled devices. The recent Cochrane systematic review by Ullman and colleagues18 found moderate quality evidence that CGI dressings reduced the frequency of catheter-related BSI per 1000 patient days compared with conventional polyurethane dressings (relative risk (RR) 0.51, 95% CI 0.33 to 0.78; p=0.002). The prevalence of catheter tip colonisation was also significantly reduced (RR=0.58; 95% CI 0.47 to 0.73; p<0.001). The transferability of these results outside of the critical care population has yet to be established, considering the different CVAD dwell times, insertion technique and clinician groups caring for CVADs in the various healthcare settings.15 18
Alternative securement and dressing options have become available that may be superior to suturing and polyurethane dressings for preventing complications, but these have not yet been adequately tested for efficacy, acceptability or cost-effectiveness.15 Sutureless securement devices (SSDs) have large adhesive padded footplates with CVAD-locking clasps of plastic or Velcro (see figure 1B). They aim to reduce movement, kinking and flow impedance15 16, and are used with polyurethane dressings. A manufacturer-sponsored randomised controlled trial (RCT) in PICCs (n=170) found significantly reduced CABSI with SSD (9.4% suture vs 1.2% SSD; p=0.04), and non-significant reduction in unplanned removal (36% suture vs 24% SSD).19 An independent RCT in dialysis devices (n=72) found reduced haematoma, thromboses and dislodgement (13.9% suture vs 8.3% SSD; p=NS).20 Neither of these studies included the paediatric population.
Integrated securement dressings (ISDs) are ‘next generation’ polyurethane dressings with a tough fabric adhesive border around the central polyurethane with continued adhesive over and underneath the CVAD body (figure 1C).15 ISDs claim to eliminate the need for a separate securement device (eg, sutures), and a reduction in costs and procedural complexity. They also include an absorbent layer around the polyurethane, which is claimed to move moisture away from the wound. This may be useful for newly inserted CVADs, which commonly ooze and require more frequent replacement, which increases CABSI risk.21 A recent adult cohort study22 (n=327 ISD; n=94 historical suture controls) reported ISD to be associated with significantly delayed onset of occlusion (from 8 to 25 days; p<0.01) in comparison to sutures.
Tissue adhesive (TA) is medical-grade ‘superglue’ (cyanoacrylate) used as an alternative to sutures in internal and external wounds.23 (figure 1D) Case reports in adults suggest TA reduces CVAD dislodgement from 12% to 4%, with no skin reactions or mechanical complications.24 25 TA is bactericidal and inhibits growth of all Gram-positive organisms (predominant in CABSI), including methicillin resistant Staphylococcus aureus (MRSA).24 TA forms an occlusive healing environment and a physical barrier to microorganisms, with haemostatic properties to reduce ooze and haematomas.24 When used with a polyurethane dressing, TA remains for 4–7 days, sloughs off slowly, and can be reapplied or removed easily with commercial wipes or petroleum jelly.26 TA may hold the key to avoiding sutures and CVAD complications by reducing pistoning, accidental removal, infection and bleeding.
These new technologies potentially reduce complications associated with the use of CVADs in the paediatric population. There are currently no strong data supporting their relative effectiveness and safety across the diverse range of CVADs and patients in paediatric clinical practice. Randomised, experimental, efficacy trials, with measures to prevent bias, are necessary to provide true estimates of relative effectiveness and inform practice.27 The UK's Medical Research Council's Developing and evaluating complex interventions framework (see figure 2)27 highlights the importance of piloting prior to undertaking large efficacy trials to prevent problems of acceptability, compliance, intervention implementation, recruitment and retention, and underpowered studies.27 Pilot studies should examine the key uncertainties that have been identified during research development. This involves testing of intervention and data collection procedures, estimating recruitment and retention numbers and determining effect estimates for future sample size calculations.
Figure 2.
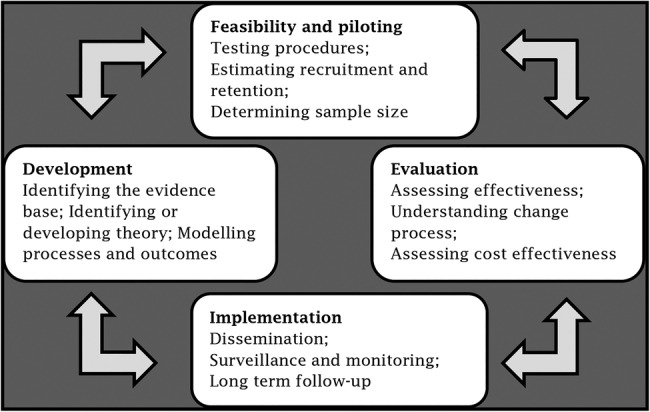
Medical Research Council framework for the evaluation of complex interventions: 27 reproduced with permission.
The primary aim of this research is to evaluate the feasibility of launching a full-scale randomised controlled efficacy trial of PICC, nt-CVAD and t-CVAD securement and dressing using predefined feasibility criteria for recruitment, retention, protocol fidelity and product acceptability. The secondary aim is to compare the effectiveness of dressings and securement products on CVAD complications and failure due to infection, occlusion, dislodgement, thrombosis, or breakage, for children in acute care facilities.
Methods and analysis
Design
Three separate pilot RCTs involving PICC, nt-CVAD and t-CVAD are being undertaken to provide information for the planning and justification of a future efficacy RCT, allowing refinement of the study components including the protocol, processes and outcomes.28 29 The trials are referred to as Central venous Access device SeCurement And Dressing Effectiveness in paediatrics (the CASCADE Junior trials).
Study setting
The three pilot RCTs were initially conducted at the Royal Children's Hospital, Brisbane, Australia; and, after local hospital mergers, the larger Lady Cilento Children's Hospital, Brisbane, Australia. These are tertiary level, specialist paediatric teaching hospitals in Queensland providing full-spectrum health services to children and young people from birth to 18 years of age. Referrals are from throughout Queensland, northern New South Wales and the Pacific Rim.
Participants
Perioperative patients requiring an elective CVAD insertion for medical treatment; or those with a non-trial CVAD in situ and requiring device replacement, as well as those requiring urgent CVAD insertion within the intensive care unit will be recruited. In total, 100 participants will be recruited to PICC-CASCADE Junior allowing 30 participants per study arm and potential 10% attrition. In total, 180 participants will be recruited to nt-CASCADE Junior allowing 55 participants per study arm and potential 10% attrition. In total, 48 participants will be recruited to t-CASCADE Junior, allowing 12 participants per study arm. As the aim of these pilot studies is to test the feasibility of the definitive RCTs, and not hypothesis testing, the power level was not a valid consideration for sample size. The CASCADE Junior pilot sample sizes are in accordance with recommendations by Thabane et al30 and Hertzog31 to facilitate accurate estimates of effect size while minimising unnecessary costs, time and recruitment of future definitive study participants.
Patients who meet all the inclusion criteria and no exclusion criteria described in table 1 are eligible for enrolment.
Table 1.
Inclusion and exclusion criteria for the CASCADE Junior trials
| Inclusion criteria | Exclusion criteria |
|---|---|
|
|
PICC-CASCADE Junior
| |
nt-CASCADE Junior
| |
t-CASCADE Junior
|
CASCADE, Central venous Access device SeCurement And Dressing Effectiveness; CVAD, central venous access device; nt, non-tunnelled; PICC, peripherally inserted central catheter; t, tunnelled.
Interventions
The intervention arms for each CVAD study have been individualised to the three device requirements (PICC, nt-CVAD and t-CVAD). Details regarding the intervention arms can be seen in box 1, with the dressing and securement technologies under evaluation illustrated in figure 1. Researchers and local clinicians developed the intervention arms; taking into consideration current local practice, best available evidence and the safety of all participants.
Box 1. Intervention arms for the CASCADE Junior trials.
PICC-CASCADE Junior
1. Standard care:
Sutureless securement device (Statlock VPPCSP ; Bard, Georgia); and
Bordered polyurethane dressing (Tegaderm 1655 or 1616; 3M, St Paul).
2. Tissue adhesive:
Tissue adhesive (Histoacryl; B. Braun, Germany); and
Bordered polyurethane dressing (Tegaderm 1655 or 1616; 3M, St Paul).
3. Integrated dressing securements:
Integrated dressing securements (SorbaView SHIELD SV353; Centurion Medical Products, Williamston).
nt-CASCADE Junior
1. Standard care:
Suture (Prolene; Ethicon, New Jersey, USA);
Chlorhexidine-impregnated disc (Biopatch 44150; Johnson & Johnson, New Jersey, USA); and
Bordered polyurethane dressing (Tegaderm 1655 or 1616; 3M, St Paul).
2. Tissue adhesive:
Suture (Prolene; Ethicon, New Jersey, USA);
Tissue adhesive (Histoacryl; B. Braun, Germany);
Chlorhexidine-impregnated disc (Biopatch 44150; Johnson & Johnson, New Jersey, USA); and
Bordered polyurethane dressing (Tegaderm 1655 or 1616; 3M, St Paul).
3. Integrated dressing securements:
Suture (Prolene; Ethicon, New Jersey, USA);
Chlorhexidine-impregnated disc (Biopatch; Johnson & Johnson, New Jersey, USA); and
Integrated dressing securements (SorbaView SHIELD SV430 or SV254; Centurion Medical Products, Williamston).
t-CASCADE Junior
1. Standard care:
Suture (Prolene; Ethicon, New Jersey, USA); and
Bordered polyurethane dressing (Tegaderm 1655 or 1616; 3M, St Paul).
2. Sutureless securement device:
Suture (Prolene; Ethicon, New Jersey, USA);
Sutureless securement device (Statlock VFDSSP; Bard, Georgia or GripLok 3601CVC; TIDI, Neenah WI); and
Bordered polyurethane dressing (Tegaderm 1655 or 1616; 3M, St Paul).
3. Tissue adhesive:
Tissue adhesive (Histoacryl; B. Braun, Germany); and
Bordered polyurethane dressing (Tegaderm 1655 or 1616; 3M, St Paul).
4. Integrated dressing-securements:
Suture (Prolene; Ethicon, New Jersey, USA); and
Integrated dressing securements (SorbaView SHIELD SV254; Centurion Medical Products, Williamston).
CASCADE, Central venous Access device SeCurement And Dressing Effectiveness; PICC, Peripherally inserted central catheter; nt, non-tunnelled; t, tunnelled.
Outcomes
Primary outcome
The primary outcome is feasibility of full efficacy trials. This will be established by composite analysis of elements of feasibility as described by Lancaster et al,28 Thabane et al30 and Hertzog.31 Full definitions of the primary and secondary outcomes are provided in box 2.
Box 2. Primary and secondary outcomes of the CASCADE Junior trials.
Primary outcome
1. Feasibility of full efficacy trials: Composite analysis of elements of feasibility:
Eligibility: ≥70% of patients screened will be eligible;
Recruitment: ≥70% of patients eligible agree to enrol;
Retention and attrition: <15% of participants are lost to follow-up or withdraw from study;
Protocol adherence: ≥80% of participants receive their allocated treatment throughout their study participation;
Missing data: <10% of data are missed during study data collection;
Satisfaction and acceptability: Parent and healthcare staff levels of satisfaction and acceptability using structured point-based questions; and
Sample size estimates: A reduction in all-cause CVAD failure or complication (defined in the secondary outcomes) by at least an absolute proportion of 5% in the experimental arms, in comparison to standard care.
Secondary outcomes
CVAD failure: Cessation of function prior to completion of therapy;10
CVAD complication: A composite of CABSI, local infection, occlusion, dislodgement, venous thrombosis or breakage (defined below);
Catheter-associated bloodstream infection (CABSI): A laboratory-confirmed bloodstream infection (LCBI) in a patient who had a central line within the 48-hour period before the development of the BSI, and that is not related to an infection at another site. The CABSI must meet one of the following criteria of LCBI: Criterion 1: Patient has a recognised pathogen cultured from one or more blood cultures and organism cultured from blood is not related to an infection at another site. OR Criterion 2: Patient has at least one of the following signs or symptoms: fever (>38°C), chills, or hypotension, and signs and symptoms and positive laboratory results are not related to an infection at another site, and common skin contaminant is cultured from two or more blood cultures drawn on separate occasions. Examples of common skin contaminants: diphtheroids (Corynebacterium spp.), Bacillus (not B. anthracis) spp., Propionibacterium spp., coagulase negative staphylococci (including S. epidermidis), viridans group streptococci, Aerococcus spp., Micrococcus spp.32 Determined by blinded infectious disease specialist;
Local infection: Purulent discharge, or redness extending 1 cm beyond the site that prompts clinician to order removal, or commence antimicrobial therapy;
Venous thrombosis: Development of thrombosed vessel (partial or complete) at the CVAD site diagnosed radiologically as requested by the treating clinician in a symptomatic patient;
Dislodgement: Partial: change in CVAD length from hub to tip, as measured by marking closest to hub, or CVAD removal because tip is no longer in superior or inferior vena cava (diagnosed by X-ray/leakage from site on injection/infusion).19 Complete: CVAD body completely leaves the vein;
Occlusion: Partial—resolved: ≥1 lumens cannot be flushed and/or aspirated, but resolves after line clearance strategy; Partial—unresolved: ≥1 lumens cannot be flushed and/or aspirated, and does not resolve after line clearance strategy; Complete: all lumens cannot be flushed and/or aspirated and does not resolve after line clearance strategy;
CVAD breakage: Visible split in CVAD material diagnosed by leakage or radiographic evidence of extravasation from a portion of the CVAD into tissue;
CVAD-related BSI: Laboratory confirmed with matched organism from blood and catheter tip culture;32
Securement-dressing failure: Replacement in under 7 days for loose, missing, bloodstained, diaphoresis or secretion soaked dressings;
CVAD and first securement dressing dwell period: Days from insertion/application of CVAD/dressing until removal;
Cost effectiveness: Estimates of direct product costs, healthcare resource utilisation (including additional equipment, staff time) and failure-associated resource usage using previously established cost estimates;33 and
Safety: Skin complications including skin rash, skin tears, blisters, pruritus, local or systemic allergic reaction.34 35
CASCADE, Central venous Access device SeCurement And Dressing Effectiveness; CVAD, central venous access device.
Study procedures
The research nurse (ReN) will screen patients daily, obtain written informed consent and undertake randomisation. The ReN will prepare study packs with securement and dressing products and will liaise closely with the CVAD insertion clinicians. Randomisation will be web-based via Griffith University https://www151.griffith.edu.au/random. This will ensure full compliance with best practice standards for randomisation generation and allocation concealment until study entry. Randomisation will be generated on a 1:1:1:1 (t-CASCADE Junior) or 1:1:1 (PICC-CASCADE and nt-CASCADE Junior) ratio for the study groups. Block size will vary randomly. The project manager will undertake quality checks to ensure allocation integrity. CVAD securement and dressings are not amenable to blinding of patients, clinical staff or ReNs.
Data collection will be facilitated using REDCap (Research Electronic Data CAPture http://project-redcap.org/) by the ReN. The ReN will visit patients daily to inspect the CVAD and dressing securement products, view medical records and talk to staff, patients and caregivers. They will collect data until 4 weeks after insertion, study withdrawal, removal of the CVAD, or hospital discharge. CVADs still in situ at 4 weeks or discharge will be censored from the study at that time. ReN will collect data on primary and secondary outcomes. Demographic data will be collected to describe the participant group and enable comparisons to inform future generalisability. Data will also be collected regarding patient and device-related characteristics that are known to increase the risk of CVAD failure.1 36–41 Variables to be collected include age, gender, diagnostic category, immunocompromise, existing infection, presence of stoma, parenteral nutrition, length of hospital stay, level of consciousness, diaphoresis, CVAD utilisation, insertion site and technique, experience of the CVAD inserter. ReN will inspect the site and collect data on all adverse events. At CVAD removal (or within 24 hours), the ReN will ask the patient or caregivers and healthcare staff about their assessment of the acceptability and satisfaction with the dressing and securement product (numeric rating scale 0–10).
CVAD procedures
The pilot studies are pragmatic in order to maximise the applicability to future efficacy trials and future generalisability, therefore, ReNs will not be involved in CVAD insertion and will minimise their involvement in CVAD care. Standardised CVAD insertions include; a large sterile drape, sterile gloves, gown and mask. The CVAD inserter will select site (eg, jugular, subclavian), CVAD type (eg, number of lumens) and approach (tunnelled or non-tunnelled) based on clinical judgement of patient needs, and then apply the allocated products.3 The ReNs will ask inserters to rate ease of application using a 11-point scale (0=very difficult, 10=very easy).
Extensive education activities and user guides will be provided to hospital staff to ensure consistency and protocol adherence. Nursing staff will change study products weekly and as clinically indicated. Product replacements/reinforcements, including tape, and the reasons for these will be recorded.
Clinical staff will take blood and CVAD tip cultures on suspicion of infection, as per standard hospital and pathology protocols.12 42 Diagnoses of CABSI and CVAD-related BSI will be made by an independent, blinded infectious diseases specialist. Similarly, ultrasound for the identification of symptomatic venous thrombosis will be requested by the clinical team coordinating the participants' care, with diagnosis made by an independent, blinded radiologist using standard department protocols.
Reliability and validity
The reliability of the CASCADE Junior trials will be ensured through the adherence to the a priori study protocol.43 Internal validity will be maintained by following the study protocol monitored by the project manager, with adherence to reporting safeguards to minimise bias. Use of computer-generated randomisation and allocation concealment will avoid risk of selection and allocation bias. The CVAD securement and dressing products being trialled are not amenable to blinding of patients, family members, clinical staff or research staff. Radiological and laboratory staff assessing the CABSI and venous thrombosis outcomes will be blinded. With an intention to treat approach, all participants will be accounted for in the final analysis following randomisation.44 The CONSORT Guidelines,45 including the checklist and diagram, will be used to report the CASCADE Junior trials findings.
Statistical methods
Each pilot study will be analysed separately. Descriptive statistics will be used to ascertain the primary outcome of feasibility for the larger trial. All randomised patients will be analysed on an intention-to-treat (ITT) basis. Comparability of groups at baseline will be assessed using clinical parameters. Incidence rates of CVAD device failure (per 1000 device days) and CVAD complication (per 100 devices) will summarise the impact of each dressing regimen; group differences will be evaluated by calculating 95% CIs and p values. CVADs in situ after 4 weeks or at hospital discharge will be censored from analysis at this point. Kaplan-Meier survival curves (with log rank test) will compare CVAD failure and complication over time. Secondary end points including dwell time, dislodgment, infection and safety will be compared between groups using parametric or non-parametric techniques as appropriate. In addition to group, multivariate regression (Cox) models will test the effect of patient and device variables associated with CVAD failure, for example, insertion site, dwell time, length of stay, diagnostic group, age, sex, mobility, comorbidities and IV medications. Prior to analysis, data cleaning of outlying figures, missing and implausible data will be undertaken, and a random 5% sample of source data will be re-entered and checked. All attempts will be made to collect the primary end point. A per-protocol analysis will assess the effect of protocol violations. p <0.1 will be evaluated as indicating some evidence against a null hypothesis, and values <0.05 will be considered statistically significant.
Estimating cost parameters
Trial costs will be collected as direct product costs (material costs) and healthcare resource utilisation (labour costs), including failure-associated costs using previously established cost estimates.33 Health resource utilisation will be measured by assessing the staff time and equipment associated with CVAD insertion (PICC, t and nt) and dressing changes.42 Group differences will be tested using a non-parametric statistical test.
Ethics and dissemination
Ethics and safety considerations
Ethics approval for the CASCADE Junior trials has been gained from the Children's Health Services Queensland (HREC/13/QRCH/181) and Griffith University (NRS/10/14/HREC) Human Research Ethics Committees (HRECs). The CASCADE Junior trials were also registered with the Australian and New Zealand Clinical Trial Registry (PICC-CASCADE ACTRN12614001327673; nt-CASCADE ACTRN12615000977572; t-CASCADE ACTRN12614000280606). Adverse events (eg, skin irritation) will be recorded and serious adverse events (eg, death) will be reported to the HRECs.
Parents/legal guardians will be given an information sheet, time to read and fully understand it, and an opportunity to ask questions. Children will be provided a youth assent form if older than 6 years of age and developmentally appropriate. All children will be provided with information regarding the study and given the opportunity to provide assent for participation. Withdrawal from the study will, in no way, affect the care they receive from the hospitals. Participant confidentiality will be ensured and anonymity guaranteed. Only aggregate data will be published and data will be stored according to National Health & Medical Research Council guidelines.46
Dissemination
In accordance with the primary outcome of feasibility, the results of this research will be used to inform the design of further efficacy RCTs of CVAD securement in paediatrics. The results of this research will also be disseminated locally at the involved children's hospital, and at relevant local, national and international vascular access and paediatric scientific meetings. Each pilot study will be separately published in a relevant healthcare journal presented in accordance with the CONSORT statement recommendations.47 Additionally, the results will be sent to the relevant organisations which lead CVAD focused clinical practice guidelines development. The funding organisations will not be involved in the analysis or preparation of publications resulting from the research.
Trial status
Recruitment of patients to the PICC-CASCADE and t-CASCADE Junior trials started in April 2014. Recruitment was paused from November 2014 to March 2015, due to the hospital merger, for the safety of all participants. Recruitment of patients to the nt-CASCADE Junior trial will commence in January 2016. It is expected that recruitment will be completed for all pilots by December 2016.
Discussion
The risk of paediatric CVAD failure and complication varies between device types.10 CVAD dressing and securement devices need to be evaluated for effectiveness and suitability across the CVAD range. A ‘one-size-fits-all’ approach to CVAD securement is inappropriate and likely to be ineffective.35 Depending on the insertion site and length, CVADs have different tensile strength requirements.15 For example, tunnelled and cuffed devices, in comparison to other CVAD types, may have lower strength requirements after tissue engraftment. PICCs may have higher strength requirements due to limb movement and device length.
The contrasting external shapes of CVADs mean some securement products may not be suitable or vary in their effectiveness to prevent complication. For example, many of the SSD products anchor devices using the CVAD ‘wings’, which are absent in tunnelled cuffed CVADs, such as Hickman or Broviac catheters. The limited skin space available to secure and dress jugular, non-tunnelled CVADs in infants and neonates can result in some securement devices also being impractical. Individual testing of CVAD securement and dressing products in paediatrics between CVAD types is necessary.
CVAD securement and dressing products provide an important contribution to the prevention of CVAD failure and complication. The ideal CVAD securement and dressing should (1) prevent accidental removal, micromotion and pistoning; (2) block bacteria entering the wound; (3) have antimicrobial properties; (4) assist with haemostasis; (5) be comfortable for patients; (6) be easy for staff to use and (7) be cost-effective. Although many alternatives to suture and polyurethane dressings exist, how these meet the above criteria is largely unknown. Systematic and narrative reviews have highlighted the dearth of literature to support practice in this area.15 48 The CASCADE Junior trials will contribute new knowledge to inform the individual efficacy of each dressing and securement type for each of the populations and devices utilising them.
Acknowledgments
The authors gratefully acknowledge the contributions of the clinicians working at the Royal Children's Hospital and Lady Cilento Children's Hospital, Brisbane who have assisted in preparing and undertaking this research.
Footnotes
Twitter: Follow Amanda Ullman at @a_ullman and Claire Rickard at @avatar_grp
Contributors: AJU conceived the study, wrote grant, developed protocol and funding applications, wrote the first draft of manuscript and approved the final draft. TK and DAL assisted with proposal development, grant application, managed the study, reviewed manuscript and approved the final draft. CMR conceived the study, wrote grant, developed protocol, setting, reviewed manuscript and approved the final draft. GM contributed to statistical methods, proposal development, reviewed the manuscript and approved the final draft. VG and TW contributed in data collection, assistance with study management and primary end point assignment, reviewed the manuscript and approved the final draft. MC contributed in grant application, prepared and reviewed the manuscript, and approved the final draft. AH and CAM contributed in grant application, oversight data collection, reviewed the manuscript and approved the final draft.
Funding: Centurion Medical Products (Williamston, USA), the Australian National Health and Medical Research Council (NHMRC) Centre for Research Excellence in Nursing Interventions for Hospitalised Patients (Brisbane, Australia), Griffith University Industry Collaboration Scheme (Brisbane, Australia) and the Centaur Memorial Fund for Nurses Scholarship have each provided partial funding for the CASCADE Junior trials. These organisations have not been involved in the design or undertaking of the study and will not be involved in the analysis or preparation of publications resulting from the research.
Competing interests: In addition to funding disclosed in the funding statement, AJU, CMR, TK have received funding through Griffith University for their research from product manufacturers (Becton Dickinson; 3M; Carefusion; Centurion Medical Products).
Patient consent: Obtained.
Ethics approval: Children's Health Services Queensland and Griffith University.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Robinson JL, Casey LM, Huynh HQ et al. . Prospective cohort study of the outcome of and risk factors for intravascular catheter-related bloodstream infections in children with intestinal failure. JPEN J Parenter Enteral Nutr 2014;38:625–30. 10.1177/0148607113517716 [DOI] [PubMed] [Google Scholar]
- 2.Intravenous Nursing New Zealand. Provisional infusion therapy standards of practice. In: O'Hara C, ed.. Auckland: Intravenous Nursing New Zealand, 2012. [Google Scholar]
- 3.Frasca D, Dahyot-Fizelier C, Mimoz O. Prevention of central venous catheter-related infection in the intensive care unit. Crit Care 2010;14:212 10.1186/cc8853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pittiruti M, Hamilton H, Biffi R et al. . ESPEN Guidelines on Parenteral Nutrition: central venous catheters (access, care, diagnosis and therapy of complications). Clin Nutr 2009;28:365–77. 10.1016/j.clnu.2009.03.015 [DOI] [PubMed] [Google Scholar]
- 5.O'Grady NP, Alexander M, Burns LA et al. . Guidelines for the prevention of intravascular catheter-related infections. Clin Infect Dis 2011;52:e162–93. 10.1093/cid/cir257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Loveday HP, Wilson JA, Pratt RJ et al. . epic3: national evidence-based guidelines for preventing healthcare-associated infections in NHS hospitals in England. J Hosp Infect 2014;86(Suppl 1):S1–70. 10.1016/S0195-6701(13)60012-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruebner R, Keren R, Coffin S et al. . Complications of central venous catheters used for the treatment of acute hematogenous osteomyelitis. Pediatrics 2006;117:1210–15. 10.1542/peds.2005-1465 [DOI] [PubMed] [Google Scholar]
- 8.Perdikaris P, Petsios K, Vasilatou-Kosmidis H et al. . Complications of Hickman-Broviac catheters in children with malignancies. Pediatr Hematol Oncol 2008;25:375–84. 10.1080/08880010802106622 [DOI] [PubMed] [Google Scholar]
- 9.Cesaro S, Corrò R, Pelosin A et al. . A prospective survey on incidence and outcome of Broviac/Hickman catheter-related complications in pediatric patients affected by hematological and oncological diseases. Ann Hematol 2004;83:183–8. 10.1007/s00277-003-0796-9 [DOI] [PubMed] [Google Scholar]
- 10.Ullman AJ, Marsh N, Mihala G et al. . Complications of central venous access devices: a systematic review. Pediatrics 2015;136:e1331–44. 10.1542/peds.2015-1507 [DOI] [PubMed] [Google Scholar]
- 11.Ross T. A survival guide for health research methods. Berkshire, UK: Open University Press, 2012. [Google Scholar]
- 12.Maki DG, Kluger DM, Crnich CJ. The risk of bloodstream infection in adults with different intravascular devices: a systematic review of 200 published prospective studies. Mayo Clin Proc 2006;81:1159–71. 10.4065/81.9.1159 [DOI] [PubMed] [Google Scholar]
- 13.Ullman AJ, Long DA, Rickard CM. Prevention of central venous catheter infections: a survey of paediatric ICU nurses’ knowledge and practice. Nurse Educ Today 2014;34:202–7. 10.1016/j.nedt.2013.09.002 [DOI] [PubMed] [Google Scholar]
- 14.Pronovost P, Needham D, Berenholtz S et al. . An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med 2006;355:2725–33. 10.1056/NEJMoa061115 [DOI] [PubMed] [Google Scholar]
- 15.Ullman AJ, Cooke M, Rickard C. Examining the role of securement and dressing products to prevent central venous access device failure: a narrative review. J Assoc Vasc Access 2015;20:99–110. 10.1016/j.java.2015.03.001 [DOI] [Google Scholar]
- 16.Frey AM, Schears GJ. Why are we stuck on tape and suture? A review of catheter securement devices. J Infus Nurs 2006;29:34–8. 10.1097/00129804-200601000-00007 [DOI] [PubMed] [Google Scholar]
- 17.Orgel E, Ji L, Pastor W et al. . Infectious morbidity by catheter type in neutropenic children with cancer. Pediatr Infect Dis J 2014;33:263–6. 10.1097/INF.0000000000000060 [DOI] [PubMed] [Google Scholar]
- 18.Ullman AJ, Cooke ML, Mitchell M et al. . Dressings and securement devices for central venous catheters (CVC). Cochrane Database Syst Rev 2015;9:CD010367 10.1002/14651858.CD010367.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamamoto AJ, Solomon JA, Soulen MC et al. . Sutureless securement device reduces complications of peripherally inserted central venous catheters. J Vasc Interv Radiol 2002;13:77–81. 10.1016/S1051-0443(07)60012-8 [DOI] [PubMed] [Google Scholar]
- 20.Teichgräber UK, de Bucourt M, Gebauer B et al. . Effectiveness of sutureless percutaneous placement of cuffed tunneled hemodialysis catheters applying StatLock attachment devices. J Vasc Access 2011;12:17–20. 10.5301/JVA.2010.6089 [DOI] [PubMed] [Google Scholar]
- 21.Timsit J, Schwebel C, Bouadma L et al. . Chlorhexidine-impregnated sponges and less frequent dressing changes for prevention of catheter related sepsis in critically ill adults: a randomized controlled trial. JAMA 2009;301:1231–41. [DOI] [PubMed] [Google Scholar]
- 22.Saijo F, Mutoh M, Kurihara M et al. . Effect of the sutureless stabilization device, SorbaView SHIELD, on the peripherally inserted central catheter. Jpn J Surg Metab Nutr 2014;48:107–13. 10.11638/jssmn.48.4_107 [DOI] [Google Scholar]
- 23.Singer AJ, Thode HCJ. A review of the literature on octylcyanoacrylate tissue adhesive. Am J Surg 2004;187: 238–48. 10.1016/j.amjsurg.2003.11.017 [DOI] [PubMed] [Google Scholar]
- 24.Wilkinson JN, Sheikh N, Jayamaha J. Tissue adhesive as an alternative to sutures for securing central venous catheters. Anaesthesia 2007;62:969–70. 10.1111/j.1365-2044.2007.05240.x [DOI] [PubMed] [Google Scholar]
- 25.Wilkinson JN, Fitz-Henry J. Securing epidural catheters with Histoacryl glue. Anaesthesia 2008;63:324 10.1111/j.1365-2044.2008.05468.x [DOI] [PubMed] [Google Scholar]
- 26.Marsh N, Webster J, Flynn J et al. . Securement methods for peripheral venous catheters: a randomised controlled pilot trial. J Vasc Access 2015;16:237–44. 10.5301/jva.5000348 [DOI] [PubMed] [Google Scholar]
- 27.Craig P, Dieppe P, Macintyre S et al. . Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008;337:a1655 10.1136/bmj.a1655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lancaster GA, Dodd S, Williamson PR. Design and analysis of pilot studies: recommendations for good practice. J Eval Clin Pract 2004;10:307–12. 10.1111/j.2002.384.doc.x [DOI] [PubMed] [Google Scholar]
- 29.Arain M, Campubell MJ, Cooper CL et al. . What is a pilot or feasibility study? A review of current practice and editorial policy. BMC Med Res Methodol 2010;10:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thabane L, Ma J, Chu R et al. . A tutorial on pilot studies: the what, why and how. BMC Med Res Methodol 2010;10:1 10.1186/1471-2288-10-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hertzog MA. Considerations in determining sample size for pilot studies. Res Nurs Health 2008;31:180–91. 10.1002/nur.20247 [DOI] [PubMed] [Google Scholar]
- 32.Centers for Disease Control and Prevention. National healthcare safety network device associated module: CLABSI. Atlanta: Government USoA, 2014:1–9. [Google Scholar]
- 33.Tuffaha H, Rickard C, Webster J et al. . Cost-effectiveness analysis of clinically indicated versus routine replacement of peripheral intravenous catheters. Appl Health Econ Heath Policy 2014;12: 51–8. 10.1007/s40258-013-0077-2 [DOI] [PubMed] [Google Scholar]
- 34.Ho KM, Litton E. Use of chlohexidine-impregnated dressing to prevent vascular and epidural catheter colonization and infection: a meta-analysis. J Antimicrob Chemother 2006;58:281–7. 10.1093/jac/dkl234 [DOI] [PubMed] [Google Scholar]
- 35.Broadhurst D, Moureau N, Ullman AJ. Central venous access devices site care practices: an international survey of 34 countries. J Vasc Access 2016;17:78–86. 10.5301/jva.5000450 [DOI] [PubMed] [Google Scholar]
- 36.Fratino G, Molinari AC, Parodi S et al. . Central venous catheter-related complications in children with oncological/hematological diseases: an observational study of 418 devices. Ann Oncol 2005;16:648–54. 10.1093/annonc/mdi111 [DOI] [PubMed] [Google Scholar]
- 37.Cecinati V, Brescia L, Tagliaferri L et al. . Catheter-related infections in pediatric patients with cancer. Eur J Clin Microbiol Infect Dis 2012;31:2869–77 10.1007/s10096-012-1652-4 [DOI] [PubMed] [Google Scholar]
- 38.Reichman DE, Greenberg JA. Reducing surgical site infections: a review. Rev Obstet Gynecol 2009;2:212–21. [PMC free article] [PubMed] [Google Scholar]
- 39.Advani S, Reich NG, Sengupta A et al. . Central line-associated bloodstream infection in hospitalized children with peripherally inserted central venous catheters: extending risk analyses outside the intensive care unit. Clin Infect Dis 2011;52:1108–15. 10.1093/cid/cir145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lorente L, Huidobro MS, Martín MM et al. . Accidental catheter removal in critically ill patients: a prospective and observational study. Crit Care 2004;8:R229–33. 10.1186/cc2874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jumani K, Advani S, Reich NG et al. . Risk factors for peripherally inserted central venous catheter complications in children. JAMA Pediatr 2013;167:429–35. 10.1001/jamapediatrics.2013.775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rickard CM, Marsh NM, Webster J et al. . Intravascular device administration sets: replacement after standard versus prolonged use in hospitalised patients-a study protocol for a randomised controlled trial (The RSVP Trial). BMJ Open 2015;5:e007257 10.1136/bmjopen-2014-007257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kimberlin CL, Winterstein AG. Validity and reliability of measurement instruments used in research. Am J Health Syst Pharm 2008;65:2276–84. 10.2146/ajhp070364 [DOI] [PubMed] [Google Scholar]
- 44.Rothwell PM. Factors that can affect the external validity of randomised controlled trials. PLoS Clin Trials 2006;1:e9 10.1371/journal.pctr.0010009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Ann Intern Med 2010;152:726–32. 10.7326/0003-4819-152-11-201006010-00232 [DOI] [PubMed] [Google Scholar]
- 46.National Health and Medical Research Council, the Australian Research Council, the Australian Vice-Chancellors’ Committee. National statement on ethical conduct in human research. Canberra: Australia Co, 2013. [Google Scholar]
- 47.Schulz KF, Altman DG, Moher D, CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. Int J Surg 2011;9:672–7. 10.1016/j.ijsu.2011.09.004 [DOI] [PubMed] [Google Scholar]
- 48.Webster J, Gillies D, O'Riordan E et al. . Gauze and tape and transparent polyurethane dressings for central venous catheters (review). Cochrane Database Syst Rev 2011;(11):CD003827 10.1002/14651858.CD003827 [DOI] [PubMed] [Google Scholar]