Abstract
Aging is the major risk factor for cardiovascular diseases (CVD), which are the leading cause of death in the United States. Traditionally, the effort to prevent CVD has been focused on addressing the conventional risk factors, including hypertension, hyperglycemia, hypercholesterolemia, and high circulating levels of triglycerides. However, recent preclinical studies have identified new approaches to combat CVD. Calorie restriction has been reproducibly shown to prolong lifespan in various experimental model animals. This has led to the development of calorie restriction mimetics and other pharmacological interventions capable to delay age-related diseases. In this review, we will address the mechanistic effects of aging per se on the cardiovascular system and focus on the pro-longevity benefits of various therapeutic strategies that support cardiovascular health.
Keywords: cardiovascular diseases, aging, calorie restriction, pharmacological strategies, prevention
I. Introduction
The population in the Western world is aging at an unprecedented rate. The substantial increase in life expectancy is associated with significant age-related cardiac, arterial and microvascular disease burden. In the United States, ischemic heart disease and stroke are the leading cause of death1 (see definition in Table 1) and their incidence exponentially increases with advanced age. Epidemiological studies clearly show that aging itself is the major risk factor for cardiovascular (CV) and cerebrovascular diseases. Yet, most of the research efforts on prevention of these diseases have ignored the mechanisms underlying cardiac and vascular effects of aging, and have focused, instead, on the development of interventions that target conventional cardiovascular risk factors (e.g. hypertension, high circulating levels of glucose, cholesterol and triglycerides). In this review, the mechanistic effects of aging per se on the CV system are considered. The possible benefits of therapeutic strategies that have the potential to improve CV function in the elderly and delay the onset of age-related CV diseases (CVD) are also discussed (see summary in Table 2 and Figure 1).
Table 1.
List of terms and their definitions
| List of terms | Definitions |
|---|---|
| Cause of death | The disease or injury, which initiated the train of morbid events leading directly to death, or the circumstances of the accident or violence which produced the fatal injury |
| Healthspan | Period of time of disease-free health |
| Lifespan | Amount of time that a person or animal actually lives |
| Morbidity | Incidence or prevalence of a disease or of all diseases |
| Mortality rate | Measure of the number of deaths in a given population |
Table 2. Pharmacological strategies to retard cardiovascular aging.
Mechanism of action of various interventions and their effects on lifespan extension, if any, and on the CV system are described.
| Intervention or compound |
Main mechanism of action |
Lifespan extension |
Effects on the CV system |
|---|---|---|---|
| Calorie Restriction |
|
|
|
| Rapamycin |
|
|
|
| Metformin |
|
|
|
| Resveratrol |
|
|
|
| SRT1720/ SRT2104 |
|
|
|
| ACE inhibitors |
|
|
|
| Aspirin |
|
|
|
| Statins |
|
|
|
| β-Blockers |
|
|
|
| AT1 blockers |
|
|
|
| Omecamtiv mecarbil |
|
|
|
| Berberine |
|
|
|
| PUFAs |
|
|
|
| Nrf2 (Nfe2l2) activators |
|
|
|
| Mito- targeted antioxidants |
|
|
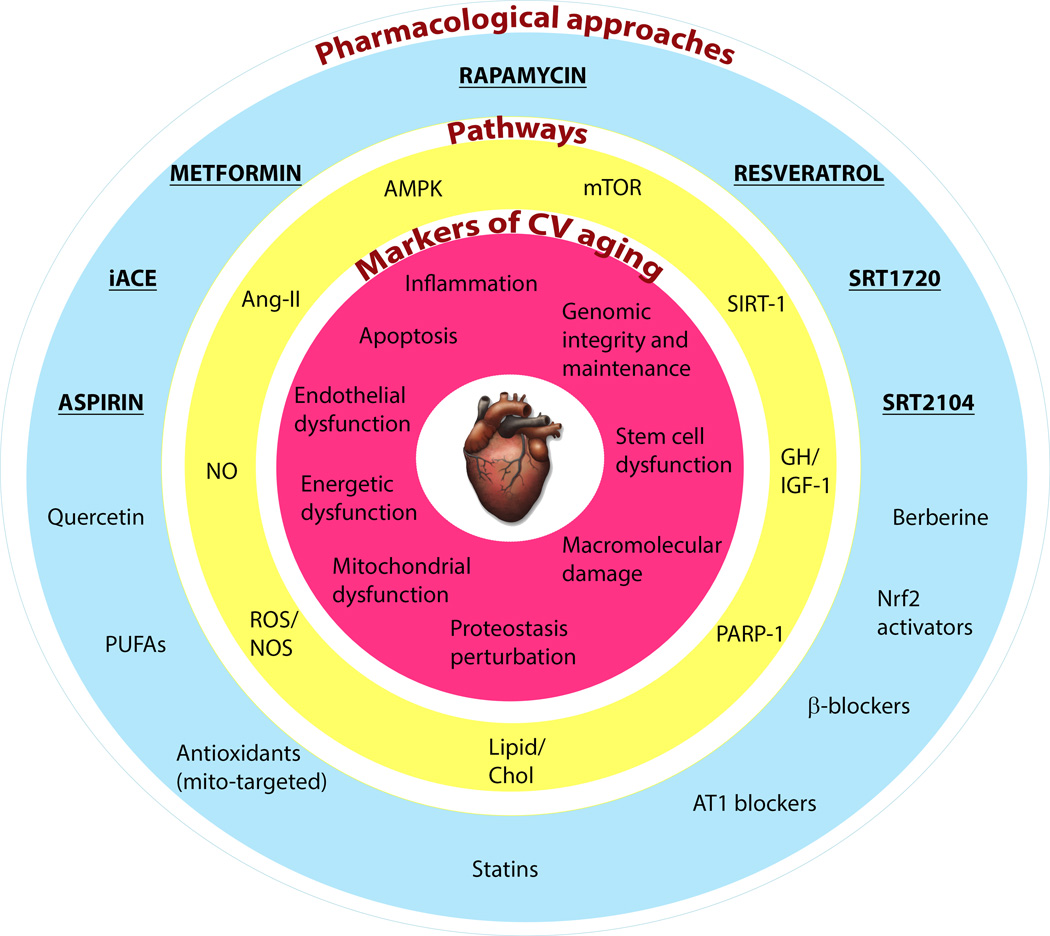
FIGURE 1. Pharmacological strategies to combat cardiovascular aging.
Age-associated changes in cardiac and vascular properties (depicted in the inner red circle) can be delayed by targeting the related pathways (in the middle yellow circle) with small molecules (represented in the outer blue circle). Some of the pharmacological strategies highlighted in the diagram (bold and underlined) have been shown to improve longevity in healthy mammals. AMPK, 5’ adenosine monophosphate-activated protein kinase; Ang-II, angiotensin II; AT1, angiotensin II receptor, type 1; Chol, cholesterol; GH, growth hormone; iACE, inhibitors of angiotensin-converting enzyme; IGF-1, insulin-like growth factor-1; mTOR, mechanistic target of rapamycin; NO, nitric oxide; NOS, nitric oxide synthase; Nrf2, NF-E2-related factor 2; PARP-1, poly (ADP-ribose) polymerase 1; PUFAs, polyunsaturated fatty acids; ROS, reactive oxygen species; SIRT-1, sirtuin (silent mating type information regulation 2 homolog) 1.
II. Mechanisms of cardiovascular aging: from oxidative stress and chronic low grade inflammation to structural and functional impairment
1) Cardiac aging
A continuum of progressive cardiac structural and functional alterations occurs with age in humans and laboratory animals, including increases in collagen levels, cardiac hypertrophy,2 decreased heart rate and diastolic filling rate,3 and impaired left ventricle function (reviewed recently in 4). The molecular and cellular mechanisms of cardiac aging involve macromolecular damage and mitochondrial oxidative stress,4–6 perturbation of proteostasis,7 age-dependent declines in autophagy and ubiquitin proteasome degradation,8,9 stem cell dysfunction,10,11 extracellular matrix remodeling,12,13 increased apoptosis,14 impaired bioavailiability of nitric oxide (NO),15 poly(ADP-ribose) polymerase 1 (PARP-1) activation and cellular energetic dysfunction,16 activation of the renin-angiotensin-aldosterone system, and age-related low-grade sterile inflammation.
2) Vascular aging
Changes in the structure and function of the large arteries that occur throughout life include diffuse intimal and medial thickening and increased stiffness of wall components, a cause of reduced distensibility of central arteries.17,18 Age-related chronic inflammation in the large arteries promote the pathogenesis of atherosclerotic diseases (stroke, peripheral artery disease, and myocardial infarction), which are a leading cause for mortality and morbidity in the elderly.
The microcirculation, with a total length of ∼100,000 km, is the most ubiquitous organ system, which envelops virtually every cell in the human body and whose age-related alterations fundamentally impact the function of every organ. The mechanisms by which microvascular alterations contribute to age-related functional decline of multiple organ systems include microvascular endothelial dysfunction,19,20 microvascular rarefaction,21,22, dysfunction of local vasoregulatory mechanisms including impaired shear stress-induced vasodilation,19 myogenic autoregulatory dysfunction,23–26 impaired microvascular functional adaptation to hypertension in the brain,24,26 disruption of microvascular barrier function (e.g. blood brain barrier disruption26,27), neurovascular uncoupling,20 activation of inflammatory processes,28–34 impaired angiogenic capacity,31,35,36 and alterations in the secretory function of microvascular endothelial cells.32,37
Studies on aged laboratory rodents, non-human primates and human subjects showed that cellular and molecular mechanisms underlying both arterial and microvascular aging include endothelial dysfunction,38 extracellular matrix remodeling, nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activation and mitochondrial oxidative stress,19,27,28,33,39–45 increased peroxynitrite production,19,46 nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) activation and up-regulation of pro-inflammatory cytokines and chemokines,30,32,42,43,47,48 NF-E2-related factor 2 (Nrf2) dysfunction and impaired cellular stress resistance,43,49,50 increased susceptibility for vascular injury,21,23,26,27,44 mitochondrial dysregulation,28,41 and endothelial senescence51 and apoptosis.30,48
III. Potential interventions that retard cardiovascular aging
While aging was historically believed to be an inevitable and intractable process, it is now well-appreciated that aging can be modulated through various environmental, life style, genetic, and pharmacological interventions. Dietary regimens and drugs that can slow the aging process continue to raise interest among the general public as well as the scientific and medical communities.52 There are already a few anti-aging interventions available that promote healthspan and/or lifespan extension and have been validated in at least three model organisms by three different laboratories. These interventions include fasting regimens, calorie restriction (the reduction in the intake of calories without malnutrition, CR), exercise, and the use of low-molecular-weight compounds, including metformin, resveratrol, and rapamycin.52
1) Cardiovascular protective effects of calorie restriction
To date, CR is the most robust intervention that has been reproducibly shown to prolong lifespan and delay the onset of age-associated diseases in both invertebrates and vertebrates, including mammals.53,54 Therefore, the successful use of pharmacological interventions that slow aging and prevent chronic disease requires an understanding of how CR delays CV aging and increases lifespan.
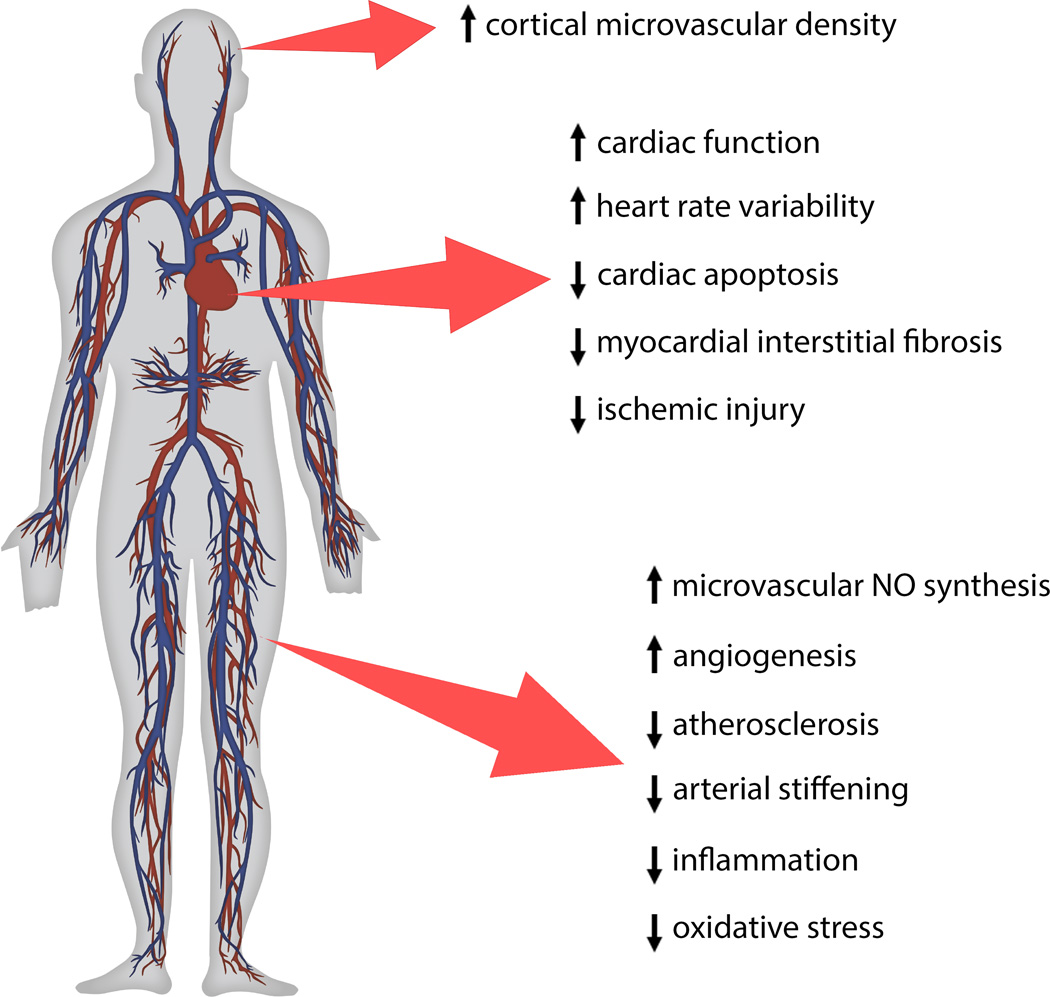
There is increasing epidemiological and experimental evidence that CR confers multifaceted CV protective effects (Figure 2) in aging and in pathological conditions associated with accelerated vascular aging.55 In laboratory animals, CR has been shown to improve endothelial function,32,34,56,57 prevent atherosclerosis and arterial stiffening,58 reduce myocardial interstitial fibrosis, cardiac apoptosis, and improve cardiac function.59 CR also confers significant microvascular protection by improving endothelial angiogenic capacity, increasing cortical microvascular density,60 and restoring microvascular NO synthesis, all of which enhancing the metabolism of parenchymal tissues.61
FIGURE 2. Anti-aging effects of caloric restriction in the cardiovascular system.
The up-arrow notation indicates an improvement or increase while the down-arrow shows decrease or impairment of cardiovascular functions and pathologies. NO, nitric oxide.
Insight into the beneficial effect of CR on several CVD and stroke risk factors in humans emanates from studies in which obese individuals were treated with some form of relatively short-term dietary restriction to lose weight. Nearly 70% of American adults are either overweight or obese, and obesity dramatically increases the risk for health problems, such as heart disease, stroke, high blood pressure (BP), type 2 diabetes and more.1 In fact, more than 2,150 Americans die from CVD each day, claiming more lives than cancer and chronic lower respiratory diseases combined.1 Therefore, weight loss offers significant improvement in the incidence of CV and metabolic disease in these individuals through reduction in body mass index, body fat, total cholesterol, serum triglyceride, inflammation, and endothelial and adipocyte dysfunction.62–64 Of significance, a diet enriched in multiple functional ingredients and concepts, i.e. natural antioxidant-rich foods, omega-3 fatty acids, prebiotics and probiotics, low-glycemic-impact foods/meals, and blood cholesterol-normalizing ingredients, reduces blood lipids and improves other cardiometabolic risk markers in healthy overweight/obese subjects in a manner that is independent of body weight reduction.65 Moreover, results from a 6-mo clinical trial show 25% CR able to reduce the estimated 10-year CVD risk66 in non-obese individuals, based on total and high-density lipoprotein (HDL) cholesterol (expressed as their ratio), systolic BP, age, and gender.67 The CALERIE Study Group has recently published a 2-year study of 25% CR in non-obese individuals that shows significant decreases in body weight, serum cholesterol, triglycerides, and mean BP without adverse events.68 The risk of coronary heart disease deaths in Asian non-obese individuals of both sexes was found to be reduced with lower energy intake.69 While high heart rate (HR) variability is associated with improved CV function, low HR variability has been linked to poor CV function.17,70 Long-term 30% CR (7 years on average) increases HR variability to a level comparable with published norms for healthy individuals 20 years younger, indicating a systemic effect that counters the expected age-associated changes in autonomic function.71
As seen in humans, studies of the effects of CR in rhesus monkeys have shown a reduction in body weight, body fat, BP, and triglyceride levels that was accompanied by improvement in glucoregulation and lipoprotein profile.54 A 50% reduction in the incidence of CVD among CR-fed monkeys has been reported by Colman and colleagues;72 however, this observation could not be replicated in a second CR study in monkeys,54 probably due to differences in diet composition and feeding protocols.
Intermittent fasting is a well-established intervention that exerts beneficial effects on many biomarkers of CV aging and risk factors for CVD in humans, including a decrease in circulating C-reactive protein.73 Intermittent fasting entails to fast on some days and feed on others, and, in doing so, reduces CV risk.74 This CR-like regimen also improves physiological CV parameters,75–77 facilitates weight loss, prevents the progression of type 2 diabetes, and appears to be cardioprotective by providing resistance to ischemic injury in rodents.78,79
Although the cellular and molecular mechanisms underlying the CV protective effects of CR regimens are still not completely understood,55 the molecular basis of CV protection relies on its beneficial effects on the different hallmarks of aging, such as metabolism, cellular oxidative stress, inflammation, autophagy, mitochondrial activity, and stem cell function.80 The existing evidence suggests that CR may improve vascular health by eliciting changes in circulating neuroendocrine factors.81 In support of this concept are studies showing that circulating factors present in the sera of CR-fed rats and non-human primates confer significant anti-oxidative, anti-inflammatory and pro-angiogenic effects in cultured endothelial cells.31,34 In addition, there is also evidence that CR activates endogenous anti-oxidative, pro-angiogenic, anti-apoptotic, and anti-inflammatory mechanisms in cell-autonomous manner, retaining a youthful phenotype in vascular cells.32 Previous studies suggest that sirtuins (see below) are key mediators of the anti-aging effects of CR,82 including its anti-oxidant and anti-inflammatory vascular effects.34 There is also important evidence that activation of Nrf2, an evolutionarily conserved transcription factor with cytoprotective and pro-survival functions, contributes to the beneficial effects of CR.32,83 The activation of adenosine monophosphate-activated protein kinase (AMPK) by mechanistic target of rapamycin (mTOR) is another key signaling pathway implicated in CR-mediated CV protection.84 Potentially, the aforementioned mechanisms that contribute to the effects of CR can be harnessed for the development of new pharmacological approaches to prevent and treat CV and cerebrovascular diseases in elderly patients.85
In response to 25–30% CR, which usually leads to a 10% decrease in body weight, and to intermittent fasting, improvement in CVD markers is observed in humans.67,68 However, more studies are needed to understand the dynamic interplay between the degree of CR and the frequency of food consumption in the modulation of metabolic and molecular pathways and prevention of CV diseases.
2) Pleiotropic cardiovascular protective effects of GH/IGF-1 axis
Growth hormone (GH) is involved in the regulation of somatic growth and development,86 and in the regulation of metabolism87 by acting directly via the GH receptor and subsequent production of insulin-like growth factor 1 (IGF-1) from the liver. The local production of IGF1 in the CV system promotes paracrine signaling88 and is associated with CV protection in humans and laboratory animals.89–91 The systemic GH and IGF-1 levels decline progressively during aging92 and, although controversial, GH and IGF-1 deficiency appear to be involved in the increased CVD risk and endothelial dysfunction.93–95 The CR-mediated increase in cardiac-specific IGF-1 expression could contribute to the paracrine CV protection.96,97 The mammalian heart has a limited amount of cardiomyocyte stem cells and this number tends to decrease with aging.98 IGF-1 overexpression is able to prevent this loss by mounting an effective response on several fronts: delay in cellular aging and death via enhanced nuclear localization of phosphoactive protein kinase B (a.k.a. AKT) and increased telomerase activity,99 protection against apoptosis100 and oxidative damage, and lower replicative senescence rate of resident stem cells.99 The use of IGF-1 as adjuvant in stem cell therapy has been demonstrated through exposure of old animals to youthful circulation –rich in circulating IGF-1 levels– by heterochronic parabiosis.101
It is well documented that GH deficiency and low circulating levels of IGF-1 significantly increase the risk for CV and cerebrovascular diseases in humans (for a review see reference 102). In addition to its effect on stem cell function, significant microvascular protection is conferred by endocrine and paracrine IGF-1 signaling.102 Microvascular dysfunction due to age-related IGF-1 deficiency has been causally linked to the pathogenesis of vascular cognitive impairment and has also implications for the pathophysiology of cardiac failure.102 Despite evidence that treatment with low doses of GH may exert beneficial effects in the CV system, the administration of supraphysiological levels of IGF-1 is accompanied with side effects (e.g. potential diabetogenic and/or pro-tumorigenic action of IGF-1) that should be carefully monitored.
3) mTOR signaling is an important modulator of the cardiovascular aging phenotype
A leading target for anti-aging interventions is the nutrient response pathway controlled by mTOR signaling.103,104 Inhibition of this pathway by CR extends lifespan and confers healthspan increase in various animal models.105–109 mTOR is a serine/threonine kinase that activates cell anabolism, especially increasing protein synthesis and cell growth, while inhibiting catabolic mechanisms, notably autophagy. mTOR associates with specific adaptor proteins to form two distinct complexes, termed mTORC-1 and mTORC-2. mTORC-1 phosphorylates S6k1 or 4EBP1 to promote mRNA translation, and Akt and AMPK are the main mTORC1 regulators. Increase in nutrient and growth factors availability stimulates Akt-mediated activation of mTOR, but suppresses AMPK function. Activation of AMPK occurs during stress or energy deprivation, thereby inhibiting mTOR.103,110 The fundamental role of mTOR signaling in metabolic regulation contributes to the biogenesis and proper functioning of the CV system.111 In fact, embryos lacking mTORC1 or mTORC2 have failed to develop.112 Genetic disruption of mTORC1 in mouse myocardium has been implicated in dilated cardiomyopathy, through activation of autophagy and apoptosis, and accumulation of 4EBP1 associated with an increase in heart failure.113 Mice deficient in raptor (component of the mTORC1 complex) had impaired metabolism at first, followed by high mortality a few weeks later due to dilated cardiomyopathy caused by increase in autophagy and apoptosis, and reduction in cardiomyocyte growth.114 Similarly, deletion of Rictor (mTORC2 complex member) is lethal for most embryos, but the surviving mice display CV abnormalities.115 However, a down-modulation of mTOR signaling confers CV benefits in the aging animals as evidenced by the fact that the mTORC-1 inhibitor rapamycin has been reported to attenuate load-induced cardiac hypertrophy, dampen the increase in myocyte cell size,116,117 and reduce ischemic injury after myocardial infarction.118,119 Furthermore, female mice supplemented with rapamycin late in life showed improved CV aging through the decrease in inflammation and hypertrophy, and higher metabolism.120
There is a progressive incidence of cardiac hypertrophy and diastolic dysfunction with advancing age as well as accumulation of protein damage mediated by oxidation and ubiquitination. Of significance, these age-associated conditions are hampered by short term CR and rapamycin treatment.104 Inhibition of mTORC1 by rapamycin confers protection against these age-related CVD, especially in the presence of metabolic disorders. In fact, mTOR appears to be dysregulated with aging, and, therefore, a partial inhibition of this pathway allows for better control of mTOR activity in CV aging.111 By acting as a regulator of cell growth and proliferation, mTOR is also responsible for stem cell exhaustion and dysfunction. So, mTOR inhibition is beneficial also for the preservation of cardiac stem cell pool that normally decreases during aging and disease.121
4) Sirtuin activation confers diverse anti-aging cardiovascular protective effects
Members of the sirtuin family of protein deacetylases are among the best-studied mediators of CR, and the contribution of SIRT1 (silent information regulator 1, after the yeast Sir2) has been the most extensively examined. The NAD+-dependent deacetylase SIRT1 is involved in several key cellular functions, including chromatin remodeling - through histone deacetylation – and gene expression, and also in cellular energy metabolism.122 The deletion of SIRT1 interferes with CR-mediated lifespan extension in yeast, worms and flies.123–125 There is strong evidence that SIRT1 exerts multifaceted anti-atherogenic,126 anti-inflammatory,127 endothelial protective,128 and cardioprotective129 effects. These findings have led to the search of small molecule activators of SIRT1 as therapeutics to improve CV health. Earlier studies have established that the natural polyphenol resveratrol was able to activate Sir2 in yeast and SIRT1 in humans, and increasing cell survival through acetylation of p53.130 In rodents, resveratrol promotes transcriptional responses comparable to CR-mediated SIRT1 activation,130 improves health and survival of mice on a high-calorie diet,131 and confers multifaceted anti-aging vascular effects (including potent mitochondrial protective and anti-inflammatory effects) and protection against atherosclerosis, hypertension, ischemia/reperfusion injury, and heart failure.20,43,132–139 Resveratrol improves cerebromicrovascular function,20 increases cerebromicrovascular density140 and prevents cerebral microhemorrhages,141 all of which likely contribute to resveratrol-mediated improvement of cognitive function in aged mice.140 Pre-clinical studies also indicate that resveratrol supplementation reduces platelet aggregation142 and improves lipid metabolism,143 while inhibiting atherosclerotic plaque formation144 and markers of oxidative stress and inflammation.145,146 It is within this context that resveratrol improves arterial stiffness in non-human primates fed high-fat, high sugar diet through decreased levels of caspase 3 and lipid peroxidation.147 However, resveratrol also elicits off-target cellular effects, whereby AMPK is activated in a SIRT1-independent fashion148–150 and phosphodiesterases inhibited nonselectively, causing a rise in intracellular cAMP levels with concomitant, sirtuin activation and improvement in age-related phenotypes.151 The redox-sensitive transcription factor Nrf2 is potently activated by resveratrol.43,135,137 The limited number of randomized clinical trials has generated controversial results on the effect of resveratrol supplementation in humans. It would appear that resveratrol is associated with lower CVD marker levels and reduced obesity at least when studies were conducted in subjects with metabolic syndrome (reviewed in 152). SRT1720 is a specific, synthetic SIRT1 activator that has demonstrated health and lifespan benefits in models of accelerated aging.153,154 There is improvement in endothelial function and attenuation in vascular oxidative stress and inflammation in SRT1720-treated mice as they age.155 SRT1720 possesses also anti-atherogenic activity.156 The polyphenol S17834, which up-regulates SIRT1, has similar anti-inflammatory and anti-atherogenic actions and exerts cardioprotection in mice with accelerated CV aging phenotypes.157–159
There are several other natural polyphenols with antioxidant, anti-inflammatory, anti-apoptotic and/or anti-senescence properties, including quercetin, kaempferol and epicatechin, which may also potentially exert beneficial effects in CV aging either alone or in combination with existing drugs. However, rigorous pre-clinical and clinical studies are needed.
5) Cardiovascular protective effects of PARP-1 inhibitors in aging
Pharmacological inhibition of the PARP pathway has emerged as a potentially important therapeutic target for aging and age-associated diseases.16,160 PARP-1 is a member of the DNA damage surveillance network. The catalytic activity of PARP-1 was reported to increase in old age due to the age-related increases in peroxynitrite-mediated DNA strand interruptions.161–163 Upon activation PARP-1 ADP-ribosylates various nuclear proteins, including transcription factors and histones, and, as a consequence, it regulates a range of cellular pathways at the transcriptional level.164,165 PARP-1 activation up-regulates NF-κB-dependent inflammatory gene expression, which is highly relevant in CV aging.166–168 PARP-1 is a NAD+-consuming enzyme that competes with SIRT1 for the same pool of NAD+. An increase in PARP-1 activity results in SIRT1 inhibition due to lower substrate availability.169 This antagonistic crosstalk between PARP-1 and SIRT1 represents a potentially important mechanism by which PARP-1 over-activation promotes age-related cardiac and vascular dysfunction. Indeed, there is evidence suggesting that inhibition of PARP-1 may confer protection against CV aging.16,46,160,163,170
6) Activation of AMPK pathway in cardiovascular aging
Studies in invertebrates have indicated in link between increase in AMPK activity and lifespan extension.171 However, the role of AMPK in the health-protective effects of CR in mammals is under debate. AMPK has been traditionally viewed as an intracellular energy switch, but is now described as a key player in maintaining physiological processes in both the heart and the vasculature.172 Expression of constitutively active AMPK mutations produces extensive remodeling of the metabolic network in order to maintain energetic homeostasis173 at the expense of developing glycogen-storage cardiomyopathy.174 A number of cellular processes that either decrease ATP levels or increase AMP concentrations promote activation of mammalian AMPK. Moreover, pharmacological interventions that include metformin, aspirin, 5-aminoimidazole-4-carboxamide riboside, statins, thiazolidinediones and the phytochemicals berberine, quercetin, and resveratrol have the ability to activate AMPK signaling175,176 by rising the (AMP+ADP)/ATP ratio as a consequence of mitochondrial electron transport and/or glycolysis inhibition. Notably, the anti-diabetic drug metformin provides protection against the development of hyperglycaemia-induced vascular disease through improvement in endothelial function.177 This biguanide exerts vasoprotection via activation of AMPK178 even though some cellular actions could be mediated in an AMPK-independent pathway.179 Resveratrol lowers BP in spontaneously hypertensive rats and reduces cardiac hypertrophy through AMPK signaling.180,181 Aspirin, also known as acetylsalicylic acid, is used at low doses as an antiplatelet drug in the prevention of vascular ischemic events and has been shown to increase lifespan in genetically heterogeneous male mice.182 This nonsteroidal anti-inflammatory drug activates AMPK183 to decrease the expression of inducible nitric oxide synthase (iNOS) and Cox-2184 and, therefore, lowers inflammation and oxidative stress. Similar protective effects have been observed with berberine.184 These results have shed light on how metformin, aspirin, and other compounds promote lifespan extension.185–187
7) Anti-aging effects of interventions that reduce oxidative stress and improve NO bioavailability
NO is a crucial factor for the health and function of the aged CV system. One of the consequences of increased oxidative stress in aging is a functional inactivation of NO,19,188–190 resulting in significant vasomotor dysfunction and contributing to vascular inflammation, atherogenesis, and cellular energetic imbalance.42 Studies on genetically NO-deficient mice have linked the impaired NO bioavailability with increased mortality and reduced lifespan potential.15,191,192 Several experimental anti-aging interventions exist (e.g., CR,32,34,61,128,193 SIRT1 activators, resveratrol,20,43,48,137–139 rapamycin,194 TNFα antibodies,40 and treatment with NADPH oxidase inhibitors or antioxidant compounds195) that improve NO bioavailability by means of increased production and/or lower NO degradation caused by oxidative stress.
The antidiabetic drug metformin has been shown to have favorable haemodynamic and rheological effects in elderly patients with CV risk factors. Infusion of the endothelial NOS (eNOS) substrate L-arginine enhances the hemodynamic effects of metformin in type 2 diabetic patients196 through increased blood flow in muscle and adipose tissue, reduction in systolic BP in response to vasoconstrictors, and improvement in acetylcholine-mediated vasodilation.197–199 Although activation of AMPK partly mediates the pleiotropic effects of metformin, studies have shown that the biguanide improves NO-mediated endothelial-dependent vasodilatation under insulin-resistant conditions177 by mechanisms linked to increased phosphorylation of eNOS and Akt via SIRT1- and AMPK-independent pathways.200 However, the ability of metformin to regulate endothelial progenitor cell differentiation201 and stimulate ischemia-induced revascularization202 depends on AMPK/eNOS signaling cascade. Metformin also has vascular anti-inflammatory properties by down-regulating NF-κB activation, caused by phosphorylation of its inhibitor IκB in the vessel wall of experimental atherogenesis in rabbits, and decreasing serum levels of high-sensitivity C-reactive protein.203
The most commonly used classes of drugs to treat obese patients have pleiotropic antioxidant properties that contribute to their beneficial effects. Studies show that statins reduce reactive oxygen species (ROS) production in cardiac muscle, which leads to an increase in mitochondrial biogenesis and phase II antioxidant enzyme system via the PGC-1 signaling pathway.204 In endothelial cells, the activation of AKT by statins results in stimulation of eNOS activity, leading to increased NO synthesis and neoangiogenesis while the increased production of endothelial NO in the central nervous system points to a role for statins in regulating sympathetic and vagal outflow, and inhibiting central angiotensin-II mechanisms.205 Clinical trial results show that statin use has been associated with lower mortality in elderly people from age 85 to 90 by providing total cholesterol-independent benefits.206
8) Anti-aging effects of mitochondria-targeted antioxidants
There is strong evidence that with advanced age mitochondrial production of ROS significantly increases in the heart207 and vasculature.208 Direct evidence supporting a critical role of mitochondrial ROS in cardiac aging was demonstrated by studies in mice that overexpress catalase targeted to the mitochondria. These mice show 18% extension of lifespan associated with protection against cardiac aging phenotypes.209,210 These observations have led to the development and testing of mitochondria-targeted antioxidants, including Mito-Q, MitoTEMPO, mitovitamin E, mitophenyltertbutyline, and SkQ1, for their potential anti-aging CV protective effects. The Szeto-Schiller (SS) compounds represent a novel class of potent mitochondria-targeted antioxidants capable of preserving mitochondrial function by scavenging H2O2, hydroxyl radical, and peroxynitrite.211,212 The tetrapeptide SS-31 has been shown to reduce ischemia reperfusion injury and reperfusion arrhythmia and better preserve myocardial function in various infarct models.213,214 Although studies on aged Apoe−/− mice show that treatment with MitoTEMPO exerts anti-atherogenic effects,215 further research is needed to test the therapeutical benefits of mitochondria-targeted antioxidants on a range of age-related CV and cerebrovascular phenotypes both in animal models of aging and elderly humans.
9) Anti-aging effects of polyunsaturated fatty acids
There are two dietary classes of essential PUFAs, the n=6 PUFAs found primarily in vegetable oils and n=3 PUFAs mainly present in marine animals or plants. Commonly referred to as omega-3 fish oils or omega-3 fatty acids, n=3 PUFAs have been shown to be beneficial in CVD as a secondary prevention and are commonly used to lower high triglyceride levels in the blood. Experimental evidences have revealed multiple underlying molecular mechanisms of action for omega-3s, which include membrane modification, ion channel attenuation, regulation of pro-inflammatory gene expression, and production of lipid mediators.216 However, the mechanism(s) that contributes the most to the cardioprotective effects of PUFAs remains to be clarified. It is imperative that further testing be performed regarding the use of omega-3 supplementation (above the accepted minimum requirement) as a mean to slow aging and reduce diseases. Indeed, pre-clinical studies have shown that long-term intake of fish oil decreases lifespan in senescence-accelerated mice217, long-lived F1 mice218 and C. elegans.219
10) Anti-aging effects of Nrf2 activators
The redox-sensitive transcription factor Nrf2 plays an evolutionarily conserved role in orchestrating cellular antioxidant defenses and maintaining redox homeostasis, ultimately impacting on health span and/or lifespan.83,220–224 Recent evidence suggests that Nrf2 also regulates the proteasome and removal of oxidized proteins.225 Nrf2 has a critical role in preserving a youthful CV phenotype and maintaining the functional integrity of the heart and the vasculature.36,226 Accumulating evidence suggests that an age-related decline in cellular Nrf2 activity results in increased cellular sensitivity to the harmful effects of ROS in the aged CV system.49,50,55 Age-associated impairment of homeostatic responses that depends on Nrf2 has been linked to exacerbation of vascular oxidative stress49,50 and inflammation,43,227 impairment of angiogenesis,36 and increased atherogenesis.226 Importantly, activation of Nrf2 is thought to contribute significantly to the beneficial effects of CR,32,83 rendering Nrf2 an attractive drug target for anti-aging interventions. Accordingly, an increasing number of experimental and clinical studies focus on the beneficial effects of compounds that activate Nrf2, such as sulforaphane, found in broccoli, and isoflavones, in animal models of age-related CV and cerebrovascular diseases.228,229 The CR-mimetic resveratrol is also a potent activator of Nrf2,43,135,137 suggesting that Nrf2 activation may also contribute to the potent anti-aging vasoprotective effects of this polyphenol.20,230
11) Disruption of angiotensin II signaling offers anti-aging effects
Angiotensin converting enzyme (ACE) inhibitors and nonpeptide blockers of angiotensin II type 1 receptor (AT1) are currently used widely to treat hypertension and cardiac heart failure. The ACE inhibitor, enalapril, does not improve longevity in healthy mice,231,232 despite the increase in heart mitochondria number and decrease in myocardial sclerosis.232 Enalapril increases rat lifespan233 and promotes NO production through activation of mitochondrial NOS activity.234 Ramipril, another ACE inhibitor, doubles the lifespan of hypertensive rats by improving cardiac function and metabolism as well as enhancing eNOS-mediated increase in endothelial function.235 Impairment in NO-dependent endothelial function in patients with Type II diabetes is aggravated by dyslipidemia and hypertension, which can be restored by ACE inhibition and weight loss.236 The generation of pro-oxidant molecules in response to angiotensin II contributes to cell oxidation and tissue damage both in normal aging and in CV and metabolic diseases.237 As predicted, targeted disruption of the Agtr1a gene that encodes AT1A has led to a marked increase of lifespan in mice.238 Long-term pharmacological inhibition of AT1 with fonsartan results in the doubling of lifespan in hypertensive rats, together with improvement in cardiac function and metabolism, and enhanced endothelial function.239 The clinical benefits of AT1 blockers can be explained by the increase in eNOS expression in the heart and carotid artery, and marked reduction in tissue ACE expression/activities.239
Non-selective beta-adrenergic blockers, widely used to treat hypertension and ischemic heart disease, have been proposed as anti-aging drugs. Metoprolol and nebivolol increase mean and maximal lifespan in flies and median lifespan in mice240 and celiprolol prevents the transition to heart failure via NO-dependent mechanisms in mice.241
The incidence of heart failure increases progressively with advanced aging. There are many treatment modalities available for heart failure associated with reduced contractile function of the myocardium. In addition to vasodilators and diuretics, which relieve cardiac workload, therapeutic approaches for heart failure include inotropic agents that increase cardiac contractility by working either through increasing the influx of calcium or modulating adrenoreceptor signaling in cardiac myocytes. Myofilament calcium sensitizers (such as omecamtiv mecarbil)242–244 represent a new class of inotropic agents that may be used in the treatment of heart failure. Omecamtiv mecarbil facilitates actin-myosin cross bridge formation, increases the number of myosin heads involved into the force generation, and stimulates myosin ATPase activity, which result in prolonged systolic ejection time and increased ejection fraction. The apparently disparate effects of omecamtiv mecarbil on myocardial oxygen consumption in animal models warrants further studies.243,245 Other emerging new treatments capable of restoring systolic function include the potentiation of cardiomyocyte contractility, increase in cardiomyocyte survival and adaptive hypertrophy, and promoting vascularization (for an excellent overview see reference246).
The lack of effective treatment options for patients with heart failure associated with age-related diastolic dysfunction is a growing clinical problem.247 To design effective therapeutic interventions it is important to understand the various age-related pathophysiological factors contributing to diastolic stiffness. Our current understanding is that age-related diastolic stiffness is due to cardiac remodeling, cardiomyocyte hypertrophy, interstitial fibrosis with increased deposition of collagen and other extracellular matrix components, decreased elastin content, matrix metalloprotease activation, redox imbalance and increased inflammation and/or impairment in active diastolic relaxation.247 Phosphorylation of the myocardial protein titin is also an important molecular determinant of cardiomyocyte stiffness,248,249 which can be potentially modulated by therapeutic interventions.250
12) Progeria and cellular senescence in cardiovascular aging
The dynamic organization of the cell nucleus is profoundly modified during growth, development and senescence. Three different diseases of accelerated aging have been associated with defects of the nuclear lamina, including Hutchinson–Gilford progeria syndrome (HGPS), Mandibuloacral Dysplasia (MADA and MADB) and atypical-Werner syndrome.251 Treatment with the mTOR inhibitor rapamycin favors recruitment of p53-binding protein 1 or 53BP1, a key player in the DNA-damage response, to the nuclear envelope and affects the levels of prelamin A in a pattern reminiscent of that observed in cells from centenarians.252 The link between mTOR pathway and nuclear lamina defects deserves further study.
Cell senescence has been proposed to have a role in CV aging because cells positive for the cyclin-dependent kinase inhibitor p16Ink4a are key drivers of an age-related cardiac phenotype that leads to lifespan shortening in mice.253 In patients with their first acute myocardial infarction, tight glycemic control reduces senescent myocyte precursor cells, thus increasing the regenerative potential of the ischemic myocardium.254 Moreover, the secretory phenotype of p16Ink4a–positive cells includes many pro-inflammatory cytokines and chemokines and matrix metalloproteinases (MMP), which are involved in tissue remodeling. It is known that MMP-9 increases with age and its deletion in aged mice alleviates cardiac fibrosis and preserves LV diastolic function by modifying the extracellular matrix response and angiogenesis.255,256 Some drugs reduce MMP-9 expression such as atorvastatin,257 Rosa hybrida extracts258 or memantine.259 Also, inhibition of chymase, an angiotensin II-forming enzyme that activates MMP-9, has been proposed as a potentially target to prevent CV diseases.260 Therefore, the therapeutic removal of senescent cells and reduction of MMP and chymase activities may be an attractive approach to improve CV aging and extend healthy lifespan.
IV. Perspectives
Although significant progress has been achieved in describing age-related alterations in cardiac and vascular function and phenotypes, the specific roles for cell-autonomous and non-cell-autonomous mechanisms involved in CV aging processes need to be elucidated further. It is critical to understand the interactions of age-related molecular mechanisms in vascular cells with both CVD pathogenesis and systemic aging processes, and to develop interventions targeting these mechanisms to retard CV aging. Several examples of such potential therapies include CR mimetics, mitochondrial protective agents and mTOR inhibitors. There is reasonable consensus that oxidative stress and inflammation play a critical role in the pathogenesis of a range of age-related CV and cerebrovascular diseases. The concept that the same evolutionarily conserved pathways (such as sirtuins and Nrf2) controlling the aging process in mammals also determine CV health through changes in ROS production, cellular and organismal sensitivity to oxidative stress and inflammatory processes, raises the question of whether pharmacological or nutritional modulation of these pathways is effective both in retarding aging and delaying the onset of age-related CVD. Compelling evidence for circulating factors that alter aging phenotypes comes from studies using heterochronic parabiosis (e.g. reversal of age-related cerebromicrovascular rarefaction261). Further understanding of the circulating factors responsible for the transposition of the aging phenotypes in young mice and the induction of youthful phenotypes in aged mice in heterochronic parabiotic pairs will guide future experimental and translational studies on novel therapeutics to treat age-related CVD and to improve healthy CV aging. Significant advances have been made in recent years toward understanding the association between cellular senescence, aging, and age-related pathologies. Studies in genetically modified mice that express a drug-activated suicide gene specifically in senescent cells suggest that senescent cell clearance can ameliorate age-related organ dysfunction.262 These findings led to the recent development of small molecule senolytic agents to decrease senescent cell burden in aging.262,263 Research efforts should also persist in these directions to fully elucidate the specific relationship between cellular senescence in development of age-related CVD and, ultimately, to determine whether senolytic agents can reduce CV morbidity and mortality in the elderly.
Supplementary Material
Acknowledgments
Sources of Funding
This work was supported by the Intramural Research Program of the NIH, National Institute on Aging, and by grants from the American Heart Association (to ZU), the National Center for Complementary and Alternative Medicine (R01-AT006526 to ZU), the National Institute on Aging (R01-AG047879 to ZU), the Arkansas Claude Pepper Older Americans Independence Center at University of Arkansas Medical Center (to ZU; P30 AG028718), the Oklahoma Center for the Advancement of Science and Technology (to ZU), and the University of Teramo (to CDG, a PhD student under the supervision of Dr Barbara Barboni, Faculty of Veterinary Medicine, University of Teramo).
Non-standard Abbreviations and Acronyms
- ACE
angiotensin converting enzyme
- AKT
protein kinase B
- AMPK
adenosine monophosphate-activated protein kinase
- AT1
angiotensin II nonpeptide type 1 receptor
- BP
blood pressure
- CR
calorie restriction
- CV
cardiovascular
- CVD
cardiovascular diseases
- eNOS
endothelial nitric oxide synthase
- GH
growth hormone
- HR
heart rate
- IGF-1
insulin-like growth factor 1
- iNOS
inducible nitric oxide synthase
- MMP
metalloproteinase
- mRNA
messenger RNA
- mTOR
mechanistic target of rapamycin
- NAD
nicotinamide adenine dinucleotide
- NADPH
nicotinamide adenine dinucleotide phosphate
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- NO
nitric oxide
- NOS
nitric oxide synthase
- Nrf2
NF-E2-related factor 2
- PARP-1
poly(ADP-ribose) polymerase 1
- PUFA
polyunsaturated fatty acid
- ROS
reactive oxygen species
- SIRT1
silent information regulator 1
- SS
Szeto-Schiller
Footnotes
Disclosures
The authors have nothing to disclose.
References
- 1.National Center for Health Statistics. Health, United States, 2014: With Special Feature on Adults Aged 55–64. Hyattsville, Md: National Center for Health Statistics; 2015. pp. 2015–1232. [PubMed] [Google Scholar]
- 2.Levy D, Anderson KM, Savage DD, Kannel WB, Christiansen JC, Castelli WP. Echocardiographically detected left ventricular hypertrophy: prevalence, risk factors The Framingham Heart Study. Ann Intern Med. 1988;108:7–13. doi: 10.7326/0003-4819-108-1-7. [DOI] [PubMed] [Google Scholar]
- 3.Schulman SP, Lakatta EG, Fleg JL, Lakatta L, Becker LC, Gerstenblith G. Age-related decline in left ventricular filling at rest and exercise. Am J Physiol. 1992;263:H1932–H1938. doi: 10.1152/ajpheart.1992.263.6.H1932. [DOI] [PubMed] [Google Scholar]
- 4.Dai DF, Rabinovitch PS, Ungvari Z. Mitochondria and cardiovascular aging. Circ Res. 2012;110:1109–1124. doi: 10.1161/CIRCRESAHA.111.246140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muscari C, Caldarera CM, Guarnieri C. Age-dependent production of mitochondrial hydrogen peroxide, lipid peroxides and fluorescent pigments in the rat heart. Basic Res Cardiol. 1990;85:172–178. doi: 10.1007/BF01906970. [DOI] [PubMed] [Google Scholar]
- 6.Sohal RS, Sohal BH. Hydrogen peroxide release by mitochondria increases during aging. Mech Ageing Dev. 1991;57:187–202. doi: 10.1016/0047-6374(91)90034-w. [DOI] [PubMed] [Google Scholar]
- 7.de Magalhães JP. From cells to ageing: a review of models and mechanisms of cellular senescence and their impact on human ageing. Exp Cell Res. 2004;300:1–10. doi: 10.1016/j.yexcr.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 8.Johnson SC, Rabinovitch PS, Kaeberlein M. mTOR is a key modulator of ageing and age-related disease. Nature. 2013;493:338–345. doi: 10.1038/nature11861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morimoto RI, Cuervo AM. Protein homeostasis and aging: taking care of proteins from the cradle to the grave. J Gerontol A Biol Sci Med Sci. 2009;64:167–170. doi: 10.1093/gerona/gln071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olivetti G, Melissari M, Capasso JM, Anversa P. Cardiomyopathy of the aging human heart Myocyte loss and reactive cellular hypertrophy. Circ Res. 1991;68:1560–1568. doi: 10.1161/01.res.68.6.1560. [DOI] [PubMed] [Google Scholar]
- 11.Goldspink DF, Burniston JG, Tan LB. Cardiomyocyte death and the ageing and failing heart. Exp Physiol. 2003;88:447–458. doi: 10.1113/eph8802549. [DOI] [PubMed] [Google Scholar]
- 12.Brooks WW, Conrad CH. Myocardial fibrosis in transforming growth factor beta(1)heterozygous mice. J Mol Cell Cardiol. 2000;32:187–195. doi: 10.1006/jmcc.1999.1065. [DOI] [PubMed] [Google Scholar]
- 13.Wang M, Zhang J, Walker SJ, Dworakowski R, Lakatta EG, Shah AM. Involvement of NADPH oxidase in age-associated cardiac remodeling. J Mol Cell Cardiol. 2010;48:765–772. doi: 10.1016/j.yjmcc.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kajstura J, Cheng W, Sarangarajan R, Li P, Li B, Nitahara JA, Chapnick S, Reiss K, Olivetti G, Anversa P. Necrotic and apoptotic myocyte cell death in the aging heart of Fischer 344 rats. Am J Physiol. 1996;271:H1215–H1228. doi: 10.1152/ajpheart.1996.271.3.H1215. [DOI] [PubMed] [Google Scholar]
- 15.Ojaimi C, Li W, Kinugawa S, Post H, Csiszar A, Pacher P, Kaley G, Hintze TH. Transcriptional basis for exercise limitation in male eNOS-knockout mice with age: heart failure and the fetal phenotype. Am J Physiol Heart Circ Physiol. 2005;289:H1399–H1407. doi: 10.1152/ajpheart.00170.2005. [DOI] [PubMed] [Google Scholar]
- 16.Pacher P, Vaslin A, Benko R, Mabley JG, Liaudet L, Hasko G, Marton A, Batkai S, Kollai M, Szabo C. A new, potent poly(ADP-ribose) polymerase inhibitor improves cardiac and vascular dysfunction associated with advanced aging. J Pharmacol Exp Ther. 2004;311:485–491. doi: 10.1124/jpet.104.069658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part II: the aging heart in health: links to heart disease. Circulation. 2003;107:346–354. doi: 10.1161/01.cir.0000048893.62841.f7. [DOI] [PubMed] [Google Scholar]
- 18.North BJ, Sinclair DA. The intersection between aging and cardiovascular disease. Circ Res. 2012;110:1097–1108. doi: 10.1161/CIRCRESAHA.111.246876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Csiszar A, Ungvari Z, Edwards JG, Kaminski PM, Wolin MS, Koller A, Kaley G. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ Res. 2002;90:1159–1166. doi: 10.1161/01.res.0000020401.61826.ea. [DOI] [PubMed] [Google Scholar]
- 20.Toth P, Tarantini S, Tucsek Z, Ashpole NM, Sosnowska D, Gautam T, Ballabh P, Koller A, Sonntag WE, Csiszar A, Ungvari ZI. Resveratrol treatment rescues neurovascular coupling in aged mice:role of improved cerebromicrovascular endothelial function and down-regulation of NADPH oxidas. Am J Physiol Heart Circ Physiol. 2014;306:H299–H308. doi: 10.1152/ajpheart.00744.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tucsek Z, Toth P, Tarantini S, Sosnowska D, Gautam T, Warrington JP, Giles CB, Wren JD, Koller A, Ballabh P, Sonntag WE, Ungvari Z, Csiszar A. Aging exacerbates obesity-induced cerebromicrovascular rarefaction, neurovascular uncoupling, and cognitive decline in mice. J Gerontol A Biol Sci Med Sci. 2014;69:1339–1352. doi: 10.1093/gerona/glu080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Riddle DR, Sonntag WE, Lichtenwalner RJ. Microvascular plasticity in aging. Ageing Res Rev. 2003;2:149–168. doi: 10.1016/s1568-1637(02)00064-8. [DOI] [PubMed] [Google Scholar]
- 23.Toth P, Tarantini S, Springo Z, Tucsek Z, Gautam T, Giles CB, Wren JD, Koller A, Sonntag WE, Csiszar A, Ungvari Z. Aging exacerbates hypertension-induced cerebral microhemorrhages in mice: role of resveratrol treatment in vasoprotection. Aging Cell. 2015 doi: 10.1111/acel.12315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Toth P, Csiszar A, Tucsek Z, Sosnowska D, Gautam T, Koller A, Schwartzman ML, Sonntag WE, Ungvari Z. Role of 20-HETE, TRPC channels, and BKCa in dysregulation of pressure-induced Ca2+ signaling and myogenic constriction of cerebral arteries in aged hypertensive mice. Am J Physiol Heart Circ Physiol. 2013;305:H1698–H1708. doi: 10.1152/ajpheart.00377.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Toth P, Tucsek Z, Tarantini S, Sosnowska D, Gautam T, Mitschelen M, Koller A, Sonntag WE, Csiszar A, Ungvari Z. IGF-1 deficiency impairs cerebral myogenic autoregulation in hypertensive mice. J Cereb Blood Flow Metab. 2014;34:1887–1897. doi: 10.1038/jcbfm.2014.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Toth P, Tucsek Z, Sosnowska D, Gautam T, Mitschelen M, Tarantini S, Deak F, Koller A, Sonntag WE, Csiszar A, Ungvari Z. Age-related autoregulatory dysfunction and cerebromicrovascular injury in mice with angiotensin II-induced hypertension. J Cereb Blood Flow Metab. 2013;33:1732–1742. doi: 10.1038/jcbfm.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tucsek Z, Toth P, Sosnowska D, Gautam T, Mitschelen M, Koller A, Szalai G, Sonntag WE, Ungvari Z, Csiszar A. Obesity in aging exacerbates blood-brain barrier disruption, neuroinflammation, and oxidative stress in the mouse hippocampus: effects on expression of genes involved in beta-amyloid generation and Alzheimer’s disease. J Gerontol A Biol Sci Med Sci. 2014;69:1212–1226. doi: 10.1093/gerona/glt177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ungvari Z, Orosz Z, Labinskyy N, Rivera A, Xiangmin Z, Smith K, Csiszar A. Increased mitochondrial H2O2 production promotes endothelial NF-kappaB activation in aged rat arteries. Am J Physiol Heart Circ Physiol. 2007;293:H37–H47. doi: 10.1152/ajpheart.01346.2006. [DOI] [PubMed] [Google Scholar]
- 29.Csiszar A, Wang M, Lakatta EG, Ungvari ZI. Inflammation and endothelial dysfunction during aging: role of NF-κB. J Appl Physiol. 2008;105:1333–1341. doi: 10.1152/japplphysiol.90470.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Csiszar A, Ungvari Z, Koller A, Edwards JG, Kaley G. Proinflammatory phenotype of coronary arteries promotes endothelial apoptosis in aging. Physiol Genomics. 2004;17:21–30. doi: 10.1152/physiolgenomics.00136.2003. [DOI] [PubMed] [Google Scholar]
- 31.Csiszar A, Sosnowska D, Tucsek Z, Gautam T, Toth P, Losonczy G, Colman RJ, Weindruch R, Anderson RM, Sonntag WE, Ungvari Z. Circulating factors induced by caloric restriction in the nonhuman primate Macaca mulatta activate angiogenic processes in endothelial cells. J Gerontol A Biol Sci Med Sci. 2013;68:235–249. doi: 10.1093/gerona/gls158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Csiszar A, Gautam T, Sosnowska D, Tarantini S, Banki E, Tucsek Z, Toth P, Losonczy G, Koller A, Reglodi D, Giles CB, Wren JD, Sonntag WE, Ungvari Z. Caloric restriction confers persistent anti-oxidative, pro-angiogenic, and anti-inflammatory effects and promotes anti-aging miRNA expression profile in cerebromicrovascular endothelial cells of aged rats. Am J Physiol Heart Circ Physiol. 2014;307:H292–H306. doi: 10.1152/ajpheart.00307.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bailey-Downs LC, Tucsek Z, Toth P, Sosnowska D, Gautam T, Sonntag WE, Csiszar A, Ungvari Z. Aging exacerbates obesity-induced oxidative stress and inflammation in perivascular adipose tissue in mice: a paracrine mechanism contributing to vascular redox dysregulation and inflammation. J Gerontol A Biol Sci Med Sci. 2013;68:780–792. doi: 10.1093/gerona/gls238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Csiszar A, Labinskyy N, Jimenez R, Pinto JT, Ballabh P, Losonczy G, Pearson KJ, de Cabo R, Ungvari Z. Anti-oxidative and anti-inflammatory vasoprotective effects of caloric restriction in aging: role of circulating factors and SIRT1. Mech Ageing Dev. 2009;130:518–527. doi: 10.1016/j.mad.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ungvari Z, Tucsek Z, Sosnowska D, Toth P, Gautam T, Podlutsky A, Csiszar A, Losonczy G, Valcarcel-Ares MN, Sonntag WE. Aging-Induced Dysregulation of Dicer1-Dependent MicroRNA Expression Impairs Angiogenic Capacity of Rat Cerebromicrovascular Endothelial Cells. J Gerontol A Biol Sci Med Sci. 2013;68:877–891. doi: 10.1093/gerona/gls242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Valcarcel-Ares MN, Gautam T, Warrington JP, Bailey-Downs L, Sosnowska D, de Cabo R, Losonczy G, Sonntag WE, Ungvari Z, Csiszar A. Disruption of Nrf2 signaling impairs angiogenic capacity of endothelial cells: implications for microvascular aging. J Gerontol A Biol Sci Med Sci. 2012;67:821–829. doi: 10.1093/gerona/glr229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Banki E, Sosnowska D, Tucsek Z, Gautam T, Toth P, Tarantini S, Tamas A, Helyes Z, Reglodi D, Sonntag WE, Csiszar A, Ungvari Z. Age-related decline of autocrine pituitary adenylate cyclase-activating polypeptide impairs angiogenic capacity of rat cerebromicrovascular endothelial cells. J Gerontol A Biol Sci Med Sci. 2015;70:665–674. doi: 10.1093/gerona/glu116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Csiszar A, Ungvari Z, Edwards JG, Kaminski PM, Wolin MS, Koller A, Kaley G. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circulation research. 2002;90:1159–1166. doi: 10.1161/01.res.0000020401.61826.ea. [DOI] [PubMed] [Google Scholar]
- 39.Csiszar A, Labinskyy N, Orosz Z, Xiangmin Z, Buffenstein R, Ungvari Z. Vascular aging in the longest-living rodent, the naked mole rat. Am J Physiol. 2007;293:H919–H927. doi: 10.1152/ajpheart.01287.2006. [DOI] [PubMed] [Google Scholar]
- 40.Csiszar A, Labinskyy N, Smith K, Rivera A, Orosz Z, Ungvari Z. Vasculoprotective effects of anti-TNFalfa treatment in aging. The American journal of pathology. 2007;170:388–698. doi: 10.2353/ajpath.2007.060708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ungvari ZI, Labinskyy N, Gupte SA, Chander PN, Edwards JG, Csiszar A. Dysregulation of mitochondrial biogenesis in vascular endothelial and smooth muscle cells of aged rats. Am J Physiol Heart Circ Physiol. 2008;294:H2121–H2128. doi: 10.1152/ajpheart.00012.2008. [DOI] [PubMed] [Google Scholar]
- 42.Ungvari Z, Kaley G, de Cabo R, Sonntag WE, Csiszar A. Mechanisms of vascular aging: new perspectives. J Gerontol A Biol Sci Med Sci. 2010;65:1028–1041. doi: 10.1093/gerona/glq113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Csiszar A, Sosnowska D, Wang M, Lakatta EG, Sonntag WE, Ungvari Z. Age-associated proinflammatory secretory phenotype in vascular smooth muscle cells from the non-human primate Macaca mulatta: reversal by resveratrol treatment. J Gerontol A Biol Sci Med Sci. 2012;67:811–820. doi: 10.1093/gerona/glr228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tucsek Z, Gautam T, Sonntag WE, Toth P, Saito H, Salomao R, Szabo C, Csiszar A, Ungvari Z. Aging Exacerbates Microvascular Endothelial Damage Induced by Circulating Factors Present in the Serum of Septic Patients. J Gerontol A Biol Sci Med Sci. 2012 doi: 10.1093/gerona/gls232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Springo Z, Tarantini S, Toth P, Tucsek Z, Koller A, Sonntag WE, Csiszar A, Ungvari Z. Aging Exacerbates Pressure-Induced Mitochondrial Oxidative Stress in Mouse Cerebral Arteries. J Gerontol A Biol Sci Med Sci. 2015;70:1355–1359. doi: 10.1093/gerona/glu244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Radovits T, Seres L, Gero D, Lin LN, Beller CJ, Chen SH, Zotkina J, Berger I, Groves JT, Szabo C, Szabo G. The peroxynitrite decomposition catalyst FP15 improves ageing-associated cardiac and vascular dysfunction. Mech Ageing Dev. 2007;128:173–181. doi: 10.1016/j.mad.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 47.Csiszar A, Ungvari Z, Koller A, Edwards JG, Kaley G. Aging-induced proinflammatory shift in cytokine expression profile in rat coronary arteries. FASEB J. 2003;17:1183–1185. doi: 10.1096/fj.02-1049fje. [DOI] [PubMed] [Google Scholar]
- 48.Pearson KJ, Baur JA, Lewis KN, et al. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 2008;8:157–168. doi: 10.1016/j.cmet.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ungvari Z, Bailey-Downs L, Sosnowska D, Gautam T, Koncz P, Losonczy G, Ballabh P, de Cabo R, Sonntag WE, Csiszar A. Vascular oxidative stress in aging: a homeostatic failure due to dysregulation of Nrf2-mediated antioxidant response. Am J Physiol Heart Circ Physiol. 2011;301:H363–H372. doi: 10.1152/ajpheart.01134.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ungvari Z, Bailey-Downs L, Gautam T, Sosnowska D, Wang M, Monticone RE, Telljohann R, Pinto JT, de Cabo R, Sonntag WE, Lakatta E, Csiszar A. Age-associated vascular oxidative stress, Nrf2 dysfunction and NF-kB activation in the non-human primate Macaca mulatta. J Gerontol A Biol Sci Med Sci. 2011;66:866–875. doi: 10.1093/gerona/glr092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Erusalimsky JD. Vascular endothelial senescence: from mechanisms to pathophysiology. J Appl Physiol. 2009;106:326–332. doi: 10.1152/japplphysiol.91353.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.de Cabo R, Carmona-Gutierrez D, Bernier M, Hall MN, Madeo F. The search for antiaging interventions: from elixirs to fasting regimens. Cell. 2014;157:1515–1526. doi: 10.1016/j.cell.2014.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Longo VD, Antebi A, Bartke A, et al. Interventions to Slow Aging in Humans: Are We Ready? Aging Cell. 2015;14:497–510. doi: 10.1111/acel.12338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mattison JA, Roth GS, Beasley TM, et al. Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature. 2012;489:318–321. doi: 10.1038/nature11432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ungvari Z, Parrado-Fernandez C, Csiszar A, de Cabo R. Mechanisms underlying caloric restriction and lifespan regulation: implications for vascular aging. Circ Res. 2008;102:519–528. doi: 10.1161/CIRCRESAHA.107.168369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Walker AE, Henson GD, Reihl KD, Nielson EI, Morgan RG, Lesniewski LA, Donato AJ. Beneficial effects of lifelong caloric restriction on endothelial function are greater in conduit arteries compared to cerebral resistance arteries. Age (Dordr) 2014;36:559–569. doi: 10.1007/s11357-013-9585-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rippe C, Lesniewski L, Connell M, LaRocca T, Donato A, Seals D. Short-term calorie restriction reverses vascular endothelial dysfunction in old mice by increasing nitric oxide and reducing oxidative stress. Aging Cell. 2010;9:304–312. doi: 10.1111/j.1474-9726.2010.00557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guo Z, Mitchell-Raymundo F, Yang H, Ikeno Y, Nelson J, Diaz V, Richardson A, Reddick R. Dietary restriction reduces atherosclerosis and oxidative stress in the aorta of apolipoprotein E-deficient mice. Mech Ageing Dev. 2002;123:1121–1131. doi: 10.1016/s0047-6374(02)00008-8. [DOI] [PubMed] [Google Scholar]
- 59.Ahmet I, Tae HJ, de Cabo R, Lakatta EG, Talan MI. Effects of calorie restriction on cardioprotection and cardiovascular health. J Mol Cell Cardiol. 2011;51:263–271. doi: 10.1016/j.yjmcc.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lynch CD, Cooney PT, Bennett SA, Thornton PL, Khan AS, Ingram RL, Sonntag WE. Effects of moderate caloric restriction on cortical microvascular density and local cerebral blood flow in aged rats. Neurobiol Aging. 1999;20:191–200. doi: 10.1016/s0197-4580(99)00032-9. [DOI] [PubMed] [Google Scholar]
- 61.Nisoli E, Tonello C, Cardile A, Cozzi V, Bracale R, Tedesco L, Falcone S, Valerio A, Cantoni O, Clementi E, Moncada S, Carruba MO. Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science. 2005;310:314–317. doi: 10.1126/science.1117728. [DOI] [PubMed] [Google Scholar]
- 62.Sackner-Bernstein J, Kanter D, Kaul S. Dietary Intervention for Overweight and Obese Adults: Comparison of Low-Carbohydrate and Low-Fat Diets A Meta-Analysis. PLoS One. 2015;10:e0139817. doi: 10.1371/journal.pone.0139817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Unick JL, Beavers D, Bond DS, Clark JM, Jakicic JM, Kitabchi AE, Knowler WC, Wadden TA, Wagenknecht LE, Wing RR. The long-term effectiveness of a lifestyle intervention in severely obese individuals. Am J Med. 2013;126:236–242. doi: 10.1016/j.amjmed.2012.10.010. 242.e231–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hu T, Yao L, Reynolds K, Whelton PK, Niu T, Li S, He J, Bazzano LA. The Effects of a Low-Carbohydrate Diet vs. a Low-Fat Diet on Novel Cardiovascular Risk Factors: A Randomized Controlled Trial. Nutrients. 2015;7:7978–7994. doi: 10.3390/nu7095377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tovar J, Johansson M, Bjorck I. A multifunctional diet improves cardiometabolic-related biomarkers independently of weight changes: an 8-week randomized controlled intervention in healthy overweight and obese subjects. Eur J Nutr. 2015 doi: 10.1007/s00394-015-1039-2. [DOI] [PubMed] [Google Scholar]
- 66.Anderson KM, Wilson PW, Odell PM, Kannel WB. An updated coronary risk profile A statement for health professionals. Circulation. 1991;83:356–362. doi: 10.1161/01.cir.83.1.356. [DOI] [PubMed] [Google Scholar]
- 67.Lefevre M, Redman LM, Heilbronn LK, Smith JV, Martin CK, Rood JC, Greenway FL, Williamson DA, Smith SR, Ravussin E. Caloric restriction alone and with exercise improves CVD risk in healthy non-obese individuals. Atherosclerosis. 2009;203:206–213. doi: 10.1016/j.atherosclerosis.2008.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ravussin E, Redman LM, Rochon J, et al. A 2-Year Randomized Controlled Trial of Human Caloric Restriction: Feasibility and Effects on Predictors of Health Span and Longevity. J Gerontol A Biol Sci Med Sci. 2015;70:1097–1104. doi: 10.1093/gerona/glv057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nagai M, Ohkubo T, Miura K, et al. Association of Total Energy Intake with 29-Year Mortality in the Japanese: NIPPON DATA80. J Atheroscler Thromb. 2016;23:339–354. doi: 10.5551/jat.29991. [DOI] [PubMed] [Google Scholar]
- 70.Zulfiqar U, Jurivich DA, Gao W, Singer DH. Relation of high heart rate variability to healthy longevity. Am J Cardiol. 2010;105:1181–1185. doi: 10.1016/j.amjcard.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 71.Stein PK, Soare A, Meyer TE, Cangemi R, Holloszy JO, Fontana L. Caloric restriction may reverse age-related autonomic decline in humans. Aging Cell. 2012;11:644–650. doi: 10.1111/j.1474-9726.2012.00825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, Allison DB, Cruzen C, Simmons HA, Kemnitz JW, Weindruch R. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325:201–204. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Brandhorst S, Choi IY, Wei M, et al. A Periodic Diet that Mimics Fasting Promotes Multi-System Regeneration, Enhanced Cognitive Performance, and Healthspan. Cell Metab. 2015;22:86–99. doi: 10.1016/j.cmet.2015.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brown JE, Mosley M, Aldred S. Intermittent fasting: a dietary intervention for prevention of diabetes and cardiovascular disease? The British Journal of Diabetes & Vascular Disease. 2013;13:68–72. [Google Scholar]
- 75.Mattson MP, Wan R. Beneficial effects of intermittent fasting and caloric restriction on the cardiovascular and cerebrovascular systems. J Nutr Biochem. 2005;16:129–137. doi: 10.1016/j.jnutbio.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 76.Fontana L, Villareal DT, Weiss EP, Racette SB, Steger-May K, Klein S, Holloszy JO. Calorie restriction or exercise: effects on coronary heart disease risk. factors A randomized, controlled trial. Am J Physiol Endocrinol Metab. 2007;293:E197–E202. doi: 10.1152/ajpendo.00102.2007. [DOI] [PubMed] [Google Scholar]
- 77.Ahmet I, Wan R, Mattson MP, Lakatta EG, Talan MI. Chronic alternate-day fasting results in reduced diastolic compliance and diminished systolic reserve in rats. J Card Fail. 2010;16:843–853. doi: 10.1016/j.cardfail.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Katare RG, Kakinuma Y, Arikawa M, Yamasaki F, Sato T. Chronic intermittent fasting improves the survival following large myocardial ischemia by activation of BDNF/VEGF/PI3K signaling pathway. J Mol Cell Cardiol. 2009;46:405–412. doi: 10.1016/j.yjmcc.2008.10.027. [DOI] [PubMed] [Google Scholar]
- 79.Wan R, Ahmet I, Brown M, Cheng A, Kamimura N, Talan M, Mattson MP. Cardioprotective effect of intermittent fasting is associated with an elevation of adiponectin levels in rats. J Nutr Biochem. 2010;21:413–417. doi: 10.1016/j.jnutbio.2009.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.de Cabo R, Furer-Galban S, Anson RM, Gilman C, Gorospe M, Lane MA. An in vitro model of caloric restriction. Exp Gerontol. 2003;38:631–639. doi: 10.1016/s0531-5565(03)00055-x. [DOI] [PubMed] [Google Scholar]
- 82.Baur JA, Ungvari Z, Minor RK, Le Couteur DG, de Cabo R. Are sirtuins viable targets for improving healthspan and lifespan? Nature reviews. 2012;11:443–461. doi: 10.1038/nrd3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pearson KJ, Lewis KN, Price NL, et al. Nrf2 mediates cancer protection but not prolongevity induced by caloric restriction. Proc Natl Acad Sci U S A. 2008;105:2325–2330. doi: 10.1073/pnas.0712162105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shinmura K, Tamaki K, Sano M, Murata M, Yamakawa H, Ishida H, Fukuda K. Impact of long-term caloric restriction on cardiac senescence: caloric restriction ameliorates cardiac diastolic dysfunction associated with aging. J Mol Cell Cardiol. 2011;50:117–127. doi: 10.1016/j.yjmcc.2010.10.018. [DOI] [PubMed] [Google Scholar]
- 85.Le Couteur DG, McLachlan AJ, Quinn RJ, Simpson SJ, de Cabo R. Aging biology and novel targets for drug discovery. J Gerontol A Biol Sci Med Sci. 2012;67:168–174. doi: 10.1093/gerona/glr095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Giustina A, Mazziotti G, Canalis E. Growth hormone, insulin-like growth factors, and the skeleton. Endocr Rev. 2008;29:535–559. doi: 10.1210/er.2007-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Moller N, Gormsen LC, Schmitz O, Lund S, Jorgensen JO, Jessen N. Free fatty acids inhibit growth hormone/signal transducer and activator of transcription-5 signaling in human muscle: a potential feedback mechanism. J Clin Endocrinol Metab. 2009;94:2204–2207. doi: 10.1210/jc.2008-2624. [DOI] [PubMed] [Google Scholar]
- 88.Ren J, Samson WK, Sowers JR. Insulin-like growth factor I as a cardiac hormone: physiological and pathophysiological implications in heart disease. J Mol Cell Cardiol. 1999;31:2049–2061. doi: 10.1006/jmcc.1999.1036. [DOI] [PubMed] [Google Scholar]
- 89.Evans LM, Davies JS, Goodfellow J, Rees JA, Scanlon MF. Endothelial dysfunction in hypopituitary adults with growth hormone deficiency. Clin Endocrinol (Oxf) 1999;50:457–464. doi: 10.1046/j.1365-2265.1999.00671.x. [DOI] [PubMed] [Google Scholar]
- 90.Denti L, Annoni V, Cattadori E, Salvagnini MA, Visioli S, Merli MF, Corradi F, Ceresini G, Valenti G, Hoffman AR, Ceda GP. Insulin-like growth factor 1 as a predictor of ischemic stroke outcome in the elderly. Am J Med. 2004;117:312–317. doi: 10.1016/j.amjmed.2004.02.049. [DOI] [PubMed] [Google Scholar]
- 91.Oflaz H, Sen F, Elitok A, Cimen AO, Onur I, Kasikcioglu E, Korkmaz S, Demirturk M, Kutluturk F, Pamukcu B, Ozbey N. Coronary flow reserve is impaired in patients with adult growth hormone (GH) deficiency. Clin Endocrinol (Oxf) 2007;66:524–529. doi: 10.1111/j.1365-2265.2007.02767.x. [DOI] [PubMed] [Google Scholar]
- 92.D’Costa AP, Ingram RL, Lenham JE, Sonntag WE. The regulation and mechanisms of action of growth hormone and insulin-like growth factor 1 during normal ageing. J Reprod Fertil Suppl. 1993;46:87–98. [PubMed] [Google Scholar]
- 93.Tomlinson JW, Holden N, Hills RK, Wheatley K, Clayton RN, Bates AS, Sheppard MC, Stewart PM. Association between premature mortality, hypopituitarism West Midlands Prospective Hypopituitary Study Group. Lancet. 2001;357:425–431. doi: 10.1016/s0140-6736(00)04006-x. [DOI] [PubMed] [Google Scholar]
- 94.Conti E, Carrozza C, Capoluongo E, Volpe M, Crea F, Zuppi C, Andreotti F. Insulin-like growth factor-1 as a vascular protective factor. Circulation. 2004;110:2260–2265. doi: 10.1161/01.CIR.0000144309.87183.FB. [DOI] [PubMed] [Google Scholar]
- 95.Rosen T, Bengtsson BA. Premature mortality due to cardiovascular disease in hypopituitarism. Lancet. 1990;336:285–288. doi: 10.1016/0140-6736(90)91812-o. [DOI] [PubMed] [Google Scholar]
- 96.Masternak MM, Al-Regaiey KA, Del Rosario Lim MM, Jimenez-Ortega V, Panici JA, Bonkowski MS, Kopchick JJ, Wang Z, Bartke A. Caloric restriction and growth hormone receptor knockout: effects on expression of genes involved in insulin action in the heart. Exp Gerontol. 2006;41:417–429. doi: 10.1016/j.exger.2006.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.D’Costa AP, Lenham JE, Ingram RL, Sonntag WE. Moderate caloric restriction increases type 1 IGF receptors and protein synthesis in aging rats. Mech Ageing Dev. 1993;71:59–71. doi: 10.1016/0047-6374(93)90035-p. [DOI] [PubMed] [Google Scholar]
- 98.Ballard VL, Edelberg JM. Stem cells and the regeneration of the aging cardiovascular system. Circ Res. 2007;100:1116–1127. doi: 10.1161/01.RES.0000261964.19115.e3. [DOI] [PubMed] [Google Scholar]
- 99.Torella D, Rota M, Nurzynska D, et al. Cardiac stem cell and myocyte aging, heart failure, and insulin-like growth factor-1 overexpression. Circ Res. 2004;94:514–524. doi: 10.1161/01.RES.0000117306.10142.50. [DOI] [PubMed] [Google Scholar]
- 100.Galvan V, Logvinova A, Sperandio S, Ichijo H, Bredesen DE. Type 1 insulin-like growth factor receptor (IGF-IR) signaling inhibits apoptosis signal-regulating kinase 1 (ASK1) J Biol Chem. 2003;278:13325–13332. doi: 10.1074/jbc.M211398200. [DOI] [PubMed] [Google Scholar]
- 101.Zhu G, Song M, Wang H, Zhao G, Yu Z, Yin Y, Zhao X, Huang L. Young environment reverses the declined activity of aged rat-derived endothelial progenitor cells: involvement of the phosphatidylinositol 3-kinase/Akt signaling pathway. Ann Vasc Surg. 2009;23:519–534. doi: 10.1016/j.avsg.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 102.Ungvari Z, Csiszar A. The emerging role of IGF-1 deficiency in cardiovascular aging: recent advances. J Gerontol A Biol Sci Med Sci. 2012;67:599–610. doi: 10.1093/gerona/gls072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dai DF, Karunadharma PP, Chiao YA, Basisty N, Crispin D, Hsieh EJ, Chen T, Gu H, Djukovic D, Raftery D, Beyer RP, MacCoss MJ, Rabinovitch PS. Altered proteome turnover and remodeling by short-term caloric restriction or rapamycin rejuvenate the aging heart. Aging Cell. 2014;13:529–539. doi: 10.1111/acel.12203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kaeberlein M, Powers RW, 3rd, Steffen KK, Westman EA, Hu D, Dang N, Kerr EO, Kirkland KT, Fields S, Kennedy BK. Regulation of yeast replicative life span by TOR and Sch9 in response to nutrients. Science. 2005;310:1193–1196. doi: 10.1126/science.1115535. [DOI] [PubMed] [Google Scholar]
- 106.Medvedik O, Lamming DW, Kim KD, Sinclair DA. MSN2 and MSN4 link calorie restriction and TOR to sirtuin-mediated lifespan extension in Saccharomyces cerevisiae. PLoS Biol. 2007;5:e261. doi: 10.1371/journal.pbio.0050261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kapahi P, Zid BM, Harper T, Koslover D, Sapin V, Benzer S. Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway. Curr Biol. 2004;14:885–890. doi: 10.1016/j.cub.2004.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jia K, Chen D, Riddle DL. The TORpathway interacts with the insulin signaling pathway to regulate C. elegans larval development, metabolism and life span. Development. 2004;131:3897–3906. doi: 10.1242/dev.01255. [DOI] [PubMed] [Google Scholar]
- 109.Vellai T, Takacs-Vellai K, Zhang Y, Kovacs AL, Orosz L, Muller F. Genetics: influence of TORkinase on lifespan in C. elegans. Nature. 2003;426:620. doi: 10.1038/426620a. [DOI] [PubMed] [Google Scholar]
- 110.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 111.Sciarretta S, Volpe M, Sadoshima J. Mammalian target of rapamycin signaling in cardiac physiology and disease. Circ Res. 2014;114:549–564. doi: 10.1161/CIRCRESAHA.114.302022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Guertin DA, Stevens DM, Thoreen CC, Burds AA, Kalaany NY, Moffat J, Brown M, Fitzgerald KJ, Sabatini DM. Ablation in mice of the mTORC components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKCalpha, but not S6K1. Dev Cell. 2006;11:859–871. doi: 10.1016/j.devcel.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 113.Zhang D, Contu R, Latronico MV, et al. MTORC1 regulates cardiac function and myocyte survival through 4E–BP1 inhibition in mice. J Clin Invest. 2010;120:2805–2816. doi: 10.1172/JCI43008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Shende P, Plaisance I, Morandi C, Pellieux C, Berthonneche C, Zorzato F, Krishnan J, Lerch R, Hall MN, Ruegg MA, Pedrazzini T, Brink M. Cardiac raptor ablation impairs adaptive hypertrophy, alters metabolic gene expression, and causes heart failure in mice. Circulation. 2011;123:1073–1082. doi: 10.1161/CIRCULATIONAHA.110.977066. [DOI] [PubMed] [Google Scholar]
- 115.Aimi F, Georgiopoulou S, Kalus I, Lehner F, Hegglin A, Limani P, Gomes de Lima VMAR, Hall MN, Lindenblatt N, Haas E, Battegay EJ, Humar R. Endothelial Rictor is crucial for midgestational development and sustained and extensive FGF2-induced neovascularization in the adult. Sci Rep. 2015;5:17705. doi: 10.1038/srep17705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Shioi T, McMullen JR, Tarnavski O, Converso K, Sherwood MC, Manning WJ, Izumo S. Rapamycin attenuates load-induced cardiac hypertrophy in mice. Circulation. 2003;107:1664–1670. doi: 10.1161/01.CIR.0000057979.36322.88. [DOI] [PubMed] [Google Scholar]
- 117.McMullen JR, Sherwood MC, Tarnavski O, Zhang L, Dorfman AL, Shioi T, Izumo S. Inhibition of mTOR signaling with rapamycin regresses established cardiac hypertrophy induced by pressure overload. Circulation. 2004;109:3050–3055. doi: 10.1161/01.CIR.0000130641.08705.45. [DOI] [PubMed] [Google Scholar]
- 118.Buss SJ, Muenz S, Riffel JH, Malekar P, Hagenmueller M, Weiss CS, Bea F, Bekeredjian R, Schinke-Braun M, Izumo S, Katus HA, Hardt SE. Beneficial effects of Mammalian target of rapamycin inhibition on left ventricular remodeling after myocardial infarction. J Am Coll Cardiol. 2009;54:2435–2446. doi: 10.1016/j.jacc.2009.08.031. [DOI] [PubMed] [Google Scholar]
- 119.Volkers M, Konstandin MH, Doroudgar S, Toko H, Quijada P, Din S, Joyo A, Ornelas L, Samse K, Thuerauf DJ, Gude N, Glembotski CC, Sussman MA. Mechanistic target of rapamycin complex 2 protects the heart from ischemic damage. Circulation. 2013;128:2132–2144. doi: 10.1161/CIRCULATIONAHA.113.003638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Flynn JM, O’Leary MN, Zambataro CA, Academia EC, Presley MP, Garrett BJ, Zykovich A, Mooney SD, Strong R, Rosen CJ, Kapahi P, Nelson MD, Kennedy BK, Melov S. Late-life rapamycin treatment reverses age-related heart dysfunction. Aging Cell. 2013;12:851–862. doi: 10.1111/acel.12109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Anversa P, Kajstura J, Rota M, Leri A. Regenerating new heart with stem cells. J Clin Invest. 2013;123:62–70. doi: 10.1172/JCI63068. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 122.Baur JA, Ungvari Z, Minor RK, Le Couteur DG, de Cabo R. Are sirtuins viable targets for improving healthspan and lifespan? Nat Rev Drug Discov. 2012;11:443–461. doi: 10.1038/nrd3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tissenbaum HA, Guarente L. Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature. 2001;410:227–230. doi: 10.1038/35065638. [DOI] [PubMed] [Google Scholar]
- 124.Bauer JH, Morris SN, Chang C, Flatt T, Wood JG, Helfand SL. dSir2 and Dmp53 interact to mediate aspects of CR-dependent lifespan extension in D. melanogaster. Aging (Albany NY) 2009;1:38–48. doi: 10.18632/aging.100001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kaeberlein M, McVey M, Guarente L. The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes Dev. 1999;13:2570–2580. doi: 10.1101/gad.13.19.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhang QJ, Wang Z, Chen HZ, Zhou S, Zheng W, Liu G, Wei YS, Cai H, Liu DP, Liang CC. Endothelium-specific overexpression of class III deacetylase SIRT1 decreases atherosclerosis in apolipoprotein E-deficient mice. Cardiovasc Res. 2008;80:191–199. doi: 10.1093/cvr/cvn224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Stein S, Schafer N, Breitenstein A, Besler C, Winnik S, Lohmann C, Heinrich K, Brokopp CE, Handschin C, Landmesser U, Tanner FC, Luscher TF, Matter CM. SIRT1 reduces endothelial activation without affecting vascular function in ApoE−/− mice. Aging (Albany NY) 2010;2:353–360. doi: 10.18632/aging.100162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mattagajasingh I, Kim CS, Naqvi A, Yamamori T, Hoffman TA, Jung SB, DeRicco J, Kasuno K, Irani K. SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proc Natl Acad Sci U S A. 2007;104:14855–14860. doi: 10.1073/pnas.0704329104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hsu CP, Zhai P, Yamamoto T, Maejima Y, Matsushima S, Hariharan N, Shao D, Takagi H, Oka S, Sadoshima J. Silent information regulator 1 protects the heart from ischemia/reperfusion. Circulation. 2010;122:2170–2182. doi: 10.1161/CIRCULATIONAHA.110.958033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Barger JL, Kayo T, Vann JM, et al. A low dose of dietary resveratrol partially mimics caloric restriction and retards aging parameters in mice. PLoS One. 2008;3:e2264. doi: 10.1371/journal.pone.0002264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Baur JA, Pearson KJ, Price NL, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhang H, Morgan B, Potter BJ, Ma L, Dellsperger KC, Ungvari ZI, Zhang C. Resveratrol Improves Left Ventricular Diastolic Relaxation in Type 2 Diabetes by Inhibiting Oxidative/Nitrative Stress. Am J Physiol Heart Circ Physiol. 2010;299:H985–H994. doi: 10.1152/ajpheart.00489.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhang H, Zhang J, Ungvari Z, Zhang C. Resveratrol improves endothelial function: role of TNFα and vascular oxidative stress. Arterioscler Thromb Vasc Biol. 2009;29:1164–1171. doi: 10.1161/ATVBAHA.109.187146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Csiszar A, Labinskyy N, Pinto JT, Ballabh P, Zhang H, Losonczy G, Pearson KJ, de Cabo R, Pacher P, Zhang C, Ungvari ZI. Resveratrol induces mitochondrial biogenesis in endothelial cells. Am J Physiol Heart Circ Physiol. 2009;297:H13–H20. doi: 10.1152/ajpheart.00368.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Csiszar A, Pinto JT, Gautam T, Kleusch C, Hoffmann B, Tucsek Z, Toth P, Sonntag WE, Ungvari Z. Resveratrol Encapsulated in Novel Fusogenic Liposomes Activates Nrf2 and Attenuates Oxidative Stress in Cerebromicrovascular Endothelial Cells From Aged Rats. J Gerontol A Biol Sci Med Sci. 2014;70:303–313. doi: 10.1093/gerona/glu029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Csiszar A, Smith K, Labinskyy N, Orosz Z, Rivera A, Ungvari Z. Resveratrol attenuates TNF-{alpha}-induced activation of coronary arterial endothelial cells: role of NF-{kappa}B inhibition. Am J Physiol. 2006;291:H1694–H1699. doi: 10.1152/ajpheart.00340.2006. [DOI] [PubMed] [Google Scholar]
- 137.Ungvari Z, Bagi Z, Feher A, Recchia FA, Sonntag WE, Pearson K, de Cabo R, Csiszar A. Resveratrol confers endothelial protection via activation of the antioxidant transcription factor Nrf2. Am J Physiol Heart Circ Physiol. 2010;299:H18–H24. doi: 10.1152/ajpheart.00260.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ungvari Z, Labinskyy N, Mukhopadhyay P, Pinto JT, Bagi Z, Ballabh P, Zhang C, Pacher P, Csiszar A. Resveratrol attenuates mitochondrial oxidative stress in coronary arterial endothelial cells. Am J Physiol Heart Circ Physiol. 2009;297:H1876–H1881. doi: 10.1152/ajpheart.00375.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ungvari Z, Orosz Z, Rivera A, Labinskyy N, Xiangmin Z, Olson S, Podlutsky A, Csiszar A. Resveratrol increases vascular oxidative stress resistance. Am J Physiol. 2007;292:H2417–H2424. doi: 10.1152/ajpheart.01258.2006. [DOI] [PubMed] [Google Scholar]
- 140.Oomen CA, Farkas E, Roman V, van der Beek EM, Luiten PG, Meerlo P. Resveratrol preserves cerebrovascular density and cognitive function in aging mice. Front Aging Neurosci. 2009;1:4. doi: 10.3389/neuro.24.004.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Toth P, Tarantini S, Springo Z, Tucsek Z, Gautam T, Giles CB, Wren JD, Koller A, Sonntag WE, Csiszar A, Ungvari Z. Aging exacerbates hypertension-induced cerebral microhemorrhages in mice: role of resveratrol treatment in vasoprotection. Aging Cell. 2015;14:400–408. doi: 10.1111/acel.12315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Gocmen AY, Burgucu D, Gumuslu S. Effect of resveratrol on platelet activation in hypercholesterolemic rats: CD40-CD40L system as a potential target. Appl Physiol Nutr Metab. 2011;36:323–330. doi: 10.1139/h11-022. [DOI] [PubMed] [Google Scholar]
- 143.Zang M, Xu S, Maitland-Toolan KA, Zuccollo A, Hou X, Jiang B, Wierzbicki M, Verbeuren TJ, Cohen RA. Polyphenols stimulate AMP-activated protein kinase, lower lipids, and inhibit accelerated atherosclerosis in diabetic LDL receptor-deficient mice. Diabetes. 2006;55:2180–2191. doi: 10.2337/db05-1188. [DOI] [PubMed] [Google Scholar]
- 144.Do GM, Kwon EY, Kim HJ, Jeon SM, Ha TY, Park T, Choi MS. Long-term effects of resveratrol supplementation on suppression of atherogenic lesion formation and cholesterol synthesis in apo E-deficient mice. Biochem Biophys Res Commun. 2008;374:55–59. doi: 10.1016/j.bbrc.2008.06.113. [DOI] [PubMed] [Google Scholar]
- 145.Guo R, Liu B, Wang K, Zhou S, Li W, Xu Y. Resveratrol ameliorates diabetic vascular inflammation and macrophage infiltration in db/db mice by inhibiting the NF-kappaB pathway. Diab Vasc Dis Res. 2014;11:92–102. doi: 10.1177/1479164113520332. [DOI] [PubMed] [Google Scholar]
- 146.Jimenez-Gomez Y, Mattison JA, Pearson KJ, et al. Resveratrol improves adipose insulin signaling and reduces the inflammatory response in adipose tissue of rhesus monkeys on high-fat, high-sugar diet. Cell Metab. 2013;18:533–545. doi: 10.1016/j.cmet.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Mattison JA, Wang M, Bernier M, et al. Resveratrol prevents high fat/sucrose diet-induced central arterial wall inflammation and stiffening in nonhuman primates. Cell Metab. 2014;20:183–190. doi: 10.1016/j.cmet.2014.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Beher D, Wu J, Cumine S, Kim KW, Lu SC, Atangan L, Wang M. Resveratrol is not a direct activator of SIRT1 enzyme activity. Chem Biol Drug Des. 2009;74:619–624. doi: 10.1111/j.1747-0285.2009.00901.x. [DOI] [PubMed] [Google Scholar]
- 149.Dasgupta B, Milbrandt J. Resveratrol stimulates AMP kinase activity in neurons. Proc Natl Acad Sci U S A. 2007;104:7217–7222. doi: 10.1073/pnas.0610068104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Canto C, Jiang LQ, Deshmukh AS, Mataki C, Coste A, Lagouge M, Zierath JR, Auwerx J. Interdependence of AMPK and SIRT1 for metabolic adaptation to fasting and exercise in skeletal muscle. Cell Metab. 2010;11:213–219. doi: 10.1016/j.cmet.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wang Z, Zhang L, Liang Y, Zhang C, Xu Z, Zhang L, Fuji R, Mu W, Li L, Jiang J, Ju Y, Wang Z. Cyclic AMP Mimics the Anti-ageing Effects of Calorie Restriction by Up-Regulating Sirtuin. Sci Rep. 2015;5:12012. doi: 10.1038/srep12012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Novelle MG, Wahl D, Dieguez C, Bernier M, de Cabo R. Resveratrol supplementation: Where are we now and where should we go? Ageing Res Rev. 2015;21:1–15. doi: 10.1016/j.arr.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Mitchell SJ, Martin-Montalvo A, Mercken EM, et al. The SIRT1 activator SRT1720 extends lifespan and improves health of mice fed a standard diet. Cell Rep. 2014;6:836–843. doi: 10.1016/j.celrep.2014.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Minor RK, Baur JA, Gomes AP, et al. SRT1720 improves survival and healthspan of obese mice. Sci Rep. 2011;1:70. doi: 10.1038/srep00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Gano LB, Donato AJ, Pasha HM, Hearon CM, Jr, Sindler AL, Seals DR. The SIRT1 activator SRT1720 reverses vascular endothelial dysfunction, excessive superoxide production, and inflammation with aging in mice. Am J Physiol Heart Circ Physiol. 2014;307:H1754–H1763. doi: 10.1152/ajpheart.00377.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Chen YX, Zhang M, Cai Y, Zhao Q, Dai W. The Sirt1 activator SRT1720 attenuates angiotensin II-induced atherosclerosis in apoE(−)/(−) mice through inhibiting vascular inflammatory response. Biochem Biophys Res Commun. 2015;465:732–738. doi: 10.1016/j.bbrc.2015.08.066. [DOI] [PubMed] [Google Scholar]
- 157.Xu S, Jiang B, Hou X, Shi C, Bachschmid MM, Zang M, Verbeuren TJ, Cohen RA. High-fat diet increases and the polyphenol, S17834, decreases acetylation of the sirtuin-1-dependent lysine-382 on p53 and apoptotic signaling in atherosclerotic lesion-prone aortic endothelium of normal mice. J Cardiovasc Pharmacol. 2011;58:263–271. doi: 10.1097/FJC.0b013e3182239eb7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Qin F, Siwik DA, Luptak I, et al. The polyphenols resveratrol and S17834 prevent the structural and functional sequelae of diet-induced metabolic heart disease in mice. Circulation. 2012;125:1757–1764. doi: 10.1161/CIRCULATIONAHA.111.067801. s1751–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Cayatte AJ, Rupin A, Oliver-Krasinski J, Maitland K, Sansilvestri-Morel P, Boussard MF, Wierzbicki M, Verbeuren TJ, Cohen RA. S17834, a new inhibitor of cell adhesion and atherosclerosis that targets nadph oxidase. Arterioscler Thromb Vasc Biol. 2001;21:1577–1584. doi: 10.1161/hq1001.096723. [DOI] [PubMed] [Google Scholar]
- 160.Pacher P, Mabley JG, Soriano FG, Liaudet L, Szabo C. Activation of poly(ADP-ribose) polymerase contributes to the endothelial dysfunction associated with hypertension and aging. Int J Mol Med. 2002;9:659–664. [PubMed] [Google Scholar]
- 161.Burkle A, Beneke S, Muiras ML. Poly(ADP-ribosyl)ation and aging. Exp Gerontol. 2004;39:1599–1601. doi: 10.1016/j.exger.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 162.Pacher P, Vaslin A, Benko R, Mabley JG, Liaudet L, Hasko G, Marton A, Batkai S, Kollai M, Szabo C. A new, potent poly(ADP-ribose) polymerase inhibitor improves cardiac and vascular dysfunction associated with advanced aging. The Journal of pharmacology and experimental therapeutics. 2004;311:485–491. doi: 10.1124/jpet.104.069658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Pacher P, Mabley JG, Soriano FG, Liaudet L, Komjati K, Szabo C. Endothelial dysfunction in aging animals: the role of poly(ADP-ribose) polymerase activation. Br J Pharmacol. 2002;135:1347–1350. doi: 10.1038/sj.bjp.0704627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ha HC, Hester LD, Snyder SH. Poly(ADP-ribose) polymerase-1 dependence of stress-induced transcription factors and associated gene expression in glia. Proc Natl Acad Sci U S A. 2002;99:3270–3275. doi: 10.1073/pnas.052712399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Carrillo A, Monreal Y, Ramirez P, Marin L, Parrilla P, Oliver FJ, Yelamos J. Transcription regulation of TNF-alpha-early response genes by poly(ADP-ribose) polymerase-1 in murine heart endothelial cells. Nucleic Acids Res. 2004;32:757–766. doi: 10.1093/nar/gkh239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Andreone TL, O’Connor M, Denenberg A, Hake PW, Zingarelli B. Poly(ADP-ribose) polymerase-1 regulates activation of activator protein-1 in murine fibroblasts. J Immunol. 2003;170:2113–2120. doi: 10.4049/jimmunol.170.4.2113. [DOI] [PubMed] [Google Scholar]
- 167.Zingarelli B, Hake PW, O’Connor M, Denenberg A, Kong S, Aronow BJ. Absence of poly(ADP-ribose)polymerase-1 alters nuclear factor-kappa B activation and gene expression of apoptosis regulators after reperfusion injury. Mol Med. 2003;9:143–153. doi: 10.2119/2003-00011.zingarelli. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hassa PO, Hottiger MO. A role of poly (ADP-ribose) polymerase in NF-kappaB transcriptional activation. Biol Chem. 1999;380:953–959. doi: 10.1515/BC.1999.118. [DOI] [PubMed] [Google Scholar]
- 169.Scheibye-Knudsen M, Mitchell SJ, Fang EF, et al. A high-fat diet and NAD(+) activate Sirt1 to rescue premature aging in cockayne syndrome. Cell Metab. 2014;20:840–855. doi: 10.1016/j.cmet.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Radovits T, Seres L, Gero D, Berger I, Szabo C, Karck M, Szabo G. Single dose treatment with PARP-inhibitor INO-1001 improves aging-associated cardiac and vascular dysfunction. Exp Gerontol. 2007;42:676–685. doi: 10.1016/j.exger.2007.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lopez-Lluch G, Navas P. Calorie restriction as an intervention in ageing. J Physiol. 2015 doi: 10.1113/JP270543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Shirwany NA, Zou MH. AMPK in cardiovascular health and disease. Acta Pharmacol Sin. 2010;31:1075–1084. doi: 10.1038/aps.2010.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Luptak I, Shen M, He H, et al. Aberrant activation of AMP-activated protein kinase remodels metabolic network in favor of cardiac glycogen storage. J Clin Invest. 2007;117:1432–1439. doi: 10.1172/JCI30658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Arad M, Benson DW, Perez-Atayde AR, McKenna WJ, Sparks EA, Kanter RJ, McGarry K, Seidman JG, Seidman CE. Constitutively active AMP kinase mutations cause glycogen storage disease mimicking hypertrophic cardiomyopathy. J Clin Invest. 2002;109:357–362. doi: 10.1172/JCI14571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Steinberg GR, Kemp BE. AMPK in Health and Disease. Physiol Rev. 2009;89:1025–1078. doi: 10.1152/physrev.00011.2008. [DOI] [PubMed] [Google Scholar]
- 176.Hwang JT, Kwon DY, Yoon SH. AMP-activated protein kinase: a potential target for the diseases prevention by natural occurring polyphenols. N Biotechnol. 2009;26:17–22. doi: 10.1016/j.nbt.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 177.Chen H, Li J, Yang O, Kong J, Lin G. Effect of metformin on insulin-resistant endothelial cell function. Oncol Lett. 2015;9:1149–1153. doi: 10.3892/ol.2015.2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Pyla R, Osman I, Pichavaram P, Hansen P, Segar L. Metformin exaggerates phenylephrine-induced AMPK phosphorylation independent of CaMKKbeta and attenuates contractile response in endothelium-denuded rat aorta. Biochem Pharmacol. 2014;92:266–279. doi: 10.1016/j.bcp.2014.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Ben Sahra I, Regazzetti C, Robert G, Laurent K, Le Marchand-Brustel Y, Auberger P, Tanti JF, Giorgetti-Peraldi S, Bost F. Metformin, independent of AMPK, induces mTOR inhibition and cell-cycle arrest through REDD1. Cancer Res. 2011;71:4366–4372. doi: 10.1158/0008-5472.CAN-10-1769. [DOI] [PubMed] [Google Scholar]
- 180.Dolinsky VW, Chakrabarti S, Pereira TJ, Oka T, Levasseur J, Beker D, Zordoky BN, Morton JS, Nagendran J, Lopaschuk GD, Davidge ST, Dyck JR. Resveratrol prevents hypertension and cardiac hypertrophy in hypertensive rats and mice. Biochim Biophys Acta. 2013;1832:1723–1733. doi: 10.1016/j.bbadis.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 181.Thandapilly SJ, Louis XL, Yang T, Stringer DM, Yu L, Zhang S, Wigle J, Kardami E, Zahradka P, Taylor C, Anderson HD, Netticadan T. Resveratrol prevents norepinephrine induced hypertrophy in adult rat cardiomyocytes, by activating NO-AMPK pathway. Eur J Pharmacol. 2011;668:217–224. doi: 10.1016/j.ejphar.2011.06.042. [DOI] [PubMed] [Google Scholar]
- 182.Strong R, Miller RA, Astle CM, Floyd RA, Flurkey K, Hensley KL, Javors MA, Leeuwenburgh C, Nelson JF, Ongini E, Nadon NL, Warner HR, Harrison DE. Nordihydroguaiaretic acid and aspirin increase lifespan of genetically heterogeneous male mice. Aging Cell. 2008;7:641–650. doi: 10.1111/j.1474-9726.2008.00414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Hawley SA, Fullerton MD, Ross FA, Schertzer JD, Chevtzoff C, Walker KJ, Peggie MW, Zibrova D, Green KA, Mustard KJ, Kemp BE, Sakamoto K, Steinberg GR, Hardie DG. The ancient drug salicylate directly activates AMP-activated protein kinase. Science. 2012;336:918–922. doi: 10.1126/science.1215327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.McCarty MF. AMPK activation--protean potential for boosting healthspan. Age (Dordr) 2014;36:641–663. doi: 10.1007/s11357-013-9595-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Martin-Montalvo A, Mercken EM, Mitchell SJ, et al. Metformin improves healthspan and lifespan in mice. Nat Commun. 2013;4:2192. doi: 10.1038/ncomms3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Hans H, Lone A, Aksenov V, Rollo CD. Impacts of metformin and aspirin on life history features and longevity of crickets: trade-offs versus cost-free life extension? Age (Dordr) 2015;37:31. doi: 10.1007/s11357-015-9769-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Danilov A, Shaposhnikov M, Shevchenko O, Zemskaya N, Zhavoronkov A, Moskalev A. Influence of non-steroidal anti-inflammatory drugs on Drosophila melanogaster longevity. Oncotarget. 2015;6:19428–19444. doi: 10.18632/oncotarget.5118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Adler A, Messina E, Sherman B, Wang Z, Huang H, Linke A, Hintze TH. NAD(P)H oxidase-generated superoxide anion accounts for reduced control of myocardial O2 consumption by NO in old Fischer 344 rats. Am J Physiol Heart Circ Physiol. 2003;285:H1015–H1022. doi: 10.1152/ajpheart.01047.2002. [DOI] [PubMed] [Google Scholar]
- 189.Sun D, Huang A, Yan EH, Wu Z, Yan C, Kaminski PM, Oury TD, Wolin MS, Kaley G. Reduced release of nitric oxide to shear stress in mesenteric arteries of aged rats. Am J Physiol Heart Circ Physiol. 2004;286:H2249–H2256. doi: 10.1152/ajpheart.00854.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Francia P, delli Gatti C, Bachschmid M, Martin-Padura I, Savoia C, Migliaccio E, Pelicci PG, Schiavoni M, Luscher TF, Volpe M, Cosentino F. Deletion of p66shc gene protects against age-related endothelial dysfunction. Circulation. 2004;110:2889–2895. doi: 10.1161/01.CIR.0000147731.24444.4D. [DOI] [PubMed] [Google Scholar]
- 191.Tsutsui M, Shimokawa H, Otsuji Y, Ueta Y, Sasaguri Y, Yanagihara N. Nitric oxide synthases and cardiovascular diseases: insights from genetically modified mice. Circ J. 2009;73:986–993. doi: 10.1253/circj.cj-09-0208. [DOI] [PubMed] [Google Scholar]
- 192.Li W, Mital S, Ojaimi C, Csiszar A, Kaley G, Hintze TH. Premature death and age-related cardiac dysfunction in male eNOS-knockout mice. J Mol Cell Cardiol. 2004;37:671–680. doi: 10.1016/j.yjmcc.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 193.Sasaki S, Higashi Y, Nakagawa K, Kimura M, Noma K, Hara K, Matsuura H, Goto C, Oshima T, Chayama K. A low-calorie diet improves endothelium-dependent vasodilation in obese patients with essential hypertension. Am J Hypertens. 2002;15:302–309. doi: 10.1016/s0895-7061(01)02322-6. [DOI] [PubMed] [Google Scholar]
- 194.Lin AL, Zheng W, Halloran JJ, et al. Chronic rapamycin restores brain vascular integrity and function through NO synthase activation and improves memory in symptomatic mice modeling Alzheimer’s disease. J Cereb Blood Flow Metab. 2013;33:1412–1421. doi: 10.1038/jcbfm.2013.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Fleenor BS, Seals DR, Zigler ML, Sindler AL. Superoxide-lowering therapy with TEMPOL reverses arterial dysfunction with aging in mice. Aging Cell. 2012;11:269–276. doi: 10.1111/j.1474-9726.2011.00783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Marfella R, Acampora R, Verrazzo G, Ziccardi P, De Rosa N, Giunta R, Giugliano D. Metformin improves hemodynamic and rheological responses to L-arginine in NIDDM patients. Diabetes Care. 1996;19:934–939. doi: 10.2337/diacare.19.9.934. [DOI] [PubMed] [Google Scholar]
- 197.Jansson PA, Gudbjornsdottir HS, Andersson OK, Lonnroth PN. The effect of metformin on adipose tissue metabolism and peripheral blood flow in subjects with NIDDM. Diabetes Care. 1996;19:160–164. doi: 10.2337/diacare.19.2.160. [DOI] [PubMed] [Google Scholar]
- 198.Sundaresan P, Lykos D, Daher A, Diamond T, Morris R, Howes LG. Comparative effects of glibenclamide and metformin on ambulatory blood pressure and cardiovascular reactivity in NIDDM. Diabetes Care. 1997;20:692–697. doi: 10.2337/diacare.20.5.692. [DOI] [PubMed] [Google Scholar]
- 199.Vitale C, Mercuro G, Cornoldi A, Fini M, Volterrani M, Rosano GM. Metformin improves endothelial function in patients with metabolic syndrome. J Intern Med. 2005;258:250–256. doi: 10.1111/j.1365-2796.2005.01531.x. [DOI] [PubMed] [Google Scholar]
- 200.Ghosh S, Lakshmanan AP, Hwang MJ, Kubba H, Mushannen A, Triggle CR, Ding H. Metformin improves endothelial function in aortic tissue and microvascular endothelial cells subjected to diabetic hyperglycaemic conditions. Biochem Pharmacol. 2015;98:412–421. doi: 10.1016/j.bcp.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 201.Li WD, Du XL, Qian AM, Hu N, Kong LS, Wei S, Li CL, Li XQ. Metformin regulates differentiation of bone marrow-derived endothelial progenitor cells via multiple mechanisms. Biochem Biophys Res Commun. 2015;465:803–809. doi: 10.1016/j.bbrc.2015.08.091. [DOI] [PubMed] [Google Scholar]
- 202.Takahashi N, Shibata R, Ouchi N, Sugimoto M, Murohara T, Komori K. Metformin stimulates ischemia-induced revascularization through an eNOS dependent pathway in the ischemic hindlimb mice model. J Vasc Surg. 2015;61:489–496. doi: 10.1016/j.jvs.2013.09.061. [DOI] [PubMed] [Google Scholar]
- 203.Li SN, Wang X, Zeng QT, Feng YB, Cheng X, Mao XB, Wang TH, Deng HP. Metformin inhibits nuclear factor kappaB activation and decreases serum high-sensitivity C-reactive protein level in experimental atherogenesis of rabbits. Heart Vessels. 2009;24:446–453. doi: 10.1007/s00380-008-1137-7. [DOI] [PubMed] [Google Scholar]
- 204.Bouitbir J, Charles AL, Echaniz-Laguna A, Kindo M, Daussin F, Auwerx J, Piquard F, Geny B, Zoll J. Opposite effects of statins on mitochondria of cardiac and skeletal muscles: a ‘mitohormesis’ mechanism involving reactive oxygen species and PGC-1. Eur Heart J. 2012;33:1397–1407. doi: 10.1093/eurheartj/ehr224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Gao L, Wang W, Li YL, Schultz HD, Liu D, Cornish KG, Zucker IH. Simvastatin therapy normalizes sympathetic neural control in experimental heart failure: roles of angiotensin II type 1 receptors and NAD(P)H oxidase. Circulation. 2005;112:1763–1770. doi: 10.1161/CIRCULATIONAHA.105.552174. [DOI] [PubMed] [Google Scholar]
- 206.Jacobs JM, Cohen A, Ein-Mor E, Stessman J. Cholesterol, statins, and longevity from age 70 to 90 years. J Am Med Dir Assoc. 2013;14:883–888. doi: 10.1016/j.jamda.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 207.Judge S, Jang YM, Smith A, Hagen T, Leeuwenburgh C. Age-associated increases in oxidative stress and antioxidant enzyme activities in cardiac interfibrillar mitochondria: implications for the mitochondrial theory of aging. FASEB J. 2005;19:419–421. doi: 10.1096/fj.04-2622fje. [DOI] [PubMed] [Google Scholar]
- 208.Ungvari ZI, Orosz Z, Labinskyy N, Rivera A, Xiangmin Z, Smith KE, Csiszar A. Increased mitochondrial H2O2 production promotes endothelial NF-kB activation in aged rat arteries. Am J Physiol Heart Circ Physiol. 2007;293:H37–H47. doi: 10.1152/ajpheart.01346.2006. [DOI] [PubMed] [Google Scholar]
- 209.Schriner SE, Linford NJ, Martin GM, Treuting P, Ogburn CE, Emond M, Coskun PE, Ladiges W, Wolf N, Van Remmen H, Wallace DC, Rabinovitch PS. Extension of murine life span by overexpression of catalase targeted to mitochondria. Science. 2005;308:1909–1911. doi: 10.1126/science.1106653. [DOI] [PubMed] [Google Scholar]
- 210.Dai DF, Santana LF, Vermulst M, Tomazela DM, Emond MJ, MacCoss MJ, Gollahon K, Martin GM, Loeb LA, Ladiges WC, Rabinovitch PS. Overexpression of catalase targeted to mitochondria attenuates murine cardiac aging. Circulation. 2009;119:2789–2797. doi: 10.1161/CIRCULATIONAHA.108.822403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Zhao K, Zhao GM, Wu D, Soong Y, Birk AV, Schiller PW, Szeto HH. Cell-permeable peptide antioxidants targeted to inner mitochondrial membrane inhibit mitochondrial swelling, oxidative cell death, and reperfusion injury. J Biol Chem. 2004;279:34682–34690. doi: 10.1074/jbc.M402999200. [DOI] [PubMed] [Google Scholar]
- 212.Szeto HH. Mitochondria-targeted cytoprotective peptides for ischemia-reperfusion injury. Antioxid Redox Signal. 2008;10:601–619. doi: 10.1089/ars.2007.1892. [DOI] [PubMed] [Google Scholar]
- 213.Dai DF, Chen T, Szeto H, Nieves-Cintron M, Kutyavin V, Santana LF, Rabinovitch PS. Mitochondrial targeted antioxidant peptide ameliorates hypertensive cardiomyopathy. J Am Coll Cardiol. 2011;58:73–82. doi: 10.1016/j.jacc.2010.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Dai DF, Hsieh EJ, Chen T, Menendez LG, Basisty NB, Tsai L, Beyer RP, Crispin DA, Shulman NJ, Szeto HH, Tian R, MacCoss MJ, Rabinovitch PS. Global proteomics and pathway analysis of pressure-overload-induced heart failure and its attenuation by mitochondrial-targeted peptides. Circ Heart Fail. 2013;6:1067–1076. doi: 10.1161/CIRCHEARTFAILURE.113.000406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Vendrov AE, Vendrov KC, Smith A, Yuan J, Sumida A, Robidoux J, Runge MS, Madamanchi NR. NOX4 NADPH Oxidase-Dependent Mitochondrial Oxidative Stress in Aging-Associated Cardiovascular Disease. Antioxid Redox Signal. 2015;23:1389–1409. doi: 10.1089/ars.2014.6221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Endo J, Arita M. Cardioprotective mechanism of omega-3 polyunsaturated fatty acids. J Cardiol. 2016;67:22–27. doi: 10.1016/j.jjcc.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 217.Tsuduki T, Honma T, Nakagawa K, Ikeda I, Miyazawa T. Long-term intake of fish oil increases oxidative stress and decreases lifespan in senescence-accelerated mice. Nutrition. 2011;27:334–337. doi: 10.1016/j.nut.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 218.Spindler SR, Mote PL, Flegal JM. Dietary supplementation with Lovaza and krill oil shortens the life span of long-lived F1 mice. Age (Dordr) 2014;36:9659. doi: 10.1007/s11357-014-9659-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Sugawara S, Honma T, Ito J, Kijima R, Tsuduki T. Fish oil changes the lifespan of Caenorhabditis elegans via lipid peroxidation. J Clin Biochem Nutr. 2013;52:139–145. doi: 10.3164/jcbn.12-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Rahman MM, Sykiotis GP, Nishimura M, Bodmer R, Bohmann D. Declining signal dependence of Nrf2-MafS-regulated gene expression correlates with aging phenotypes. Aging Cell. 2013;12:554–562. doi: 10.1111/acel.12078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Lewis KN, Mele J, Hayes JD, Buffenstein R. Nrf2, a Guardian of Healthspan and Gatekeeper of Species Longevity. Integr Comp Biol. 2010;50:829–843. doi: 10.1093/icb/icq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Staab TA, Griffen TC, Corcoran C, Evgrafov O, Knowles JA, Sieburth D. The conserved SKN-1/Nrf2 stress response pathway regulates synaptic function in Caenorhabditis elegans. PLoS Genet. 2013;9:e1003354. doi: 10.1371/journal.pgen.1003354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Suh JH, Shenvi SV, Dixon BM, Liu H, Jaiswal AK, Liu RM, Hagen TM. Decline in transcriptional activity of Nrf2 causes age-related loss of glutathione synthesis, which is reversible with lipoic acid. Proc Natl Acad Sci U S A. 2004;101:3381–3386. doi: 10.1073/pnas.0400282101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Sykiotis GP, Bohmann D. Keap1/Nrf2 signaling regulates oxidative stress tolerance and lifespan in Drosophila. Dev Cell. 2008;14:76–85. doi: 10.1016/j.devcel.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Chapple SJ, Siow RC, Mann GE. Crosstalk between Nrf2 and the proteasome: therapeutic potential of Nrf2 inducers in vascular disease and aging. Int J Biochem Cell Biol. 2012;44:1315–1320. doi: 10.1016/j.biocel.2012.04.021. [DOI] [PubMed] [Google Scholar]
- 226.Collins AR, Lyon CJ, Xia X, Liu JZ, Tangirala RK, Yin F, Boyadjian R, Bikineyeva A, Pratico D, Harrison DG, Hsueh WA. Age-accelerated atherosclerosis correlates with failure to upregulate antioxidant genes. Circ Res. 2009;104:e42–e54. doi: 10.1161/CIRCRESAHA.108.188771. [DOI] [PubMed] [Google Scholar]
- 227.Morrison CD, Pistell PJ, Ingram DK, Johnson WD, Liu Y, Fernandez-Kim SO, White CL, Purpera MN, Uranga RM, Bruce-Keller AJ, Keller JN. High fat diet increases hippocampal oxidative stress and cognitive impairment in aged mice: implications for decreased Nrf2 signaling. J Neurochem. 2010;114:1581–1589. doi: 10.1111/j.1471-4159.2010.06865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Alfieri A, Srivastava S, Siow RC, Modo M, Fraser PA, Mann GE. Targeting the Nrf2-Keap1 antioxidant defence pathway for neurovascular protection in stroke. J Physiol. 2011;589:4125–4136. doi: 10.1113/jphysiol.2011.210294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Tan SM, Sharma A, Stefanovic N, Yuen DY, Karagiannis TC, Meyer C, Ward KW, Cooper ME, de Haan JB. Derivative of bardoxolone methyl, dh404, in an inverse dose-dependent manner lessens diabetes-associated atherosclerosis and improves diabetic kidney disease. Diabetes. 2014;63:3091–3103. doi: 10.2337/db13-1743. [DOI] [PubMed] [Google Scholar]
- 230.Pearson KJ, Baur JA, Lewis KN, et al. Resveratrol Delays Age-Related Deterioration and Mimics Transcriptional Aspects of Dietary Restriction without Extending Life Span. Cell metabolism. 2008;8:157–168. doi: 10.1016/j.cmet.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, Pahor M, Javors MA, Fernandez E, Miller RA. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Ferder L, Inserra F, Romano L, Ercole L, Pszenny V. Effects of angiotensin-converting enzyme inhibition on mitochondrial number in the aging mouse. Am J Physiol. 1993;265:C15–C18. doi: 10.1152/ajpcell.1993.265.1.C15. [DOI] [PubMed] [Google Scholar]
- 233.Santos EL, de Picoli Souza K, da Silva ED, Batista EC, Martins PJ, D’Almeida V, Pesquero JB. Long term treatment with ACE inhibitor enalapril decreases body weight gain and increases life span in rats. Biochem Pharmacol. 2009;78:951–958. doi: 10.1016/j.bcp.2009.06.018. [DOI] [PubMed] [Google Scholar]
- 234.Boveris A, D’Amico G, Lores-Arnaiz S, Costa LE. Enalapril increases mitochondrial nitric oxide synthase activity in heart and liver. Antioxid Redox Signal. 2003;5:691–697. doi: 10.1089/152308603770379982. [DOI] [PubMed] [Google Scholar]
- 235.Linz W, Jessen T, Becker RH, Scholkens BA, Wiemer G. Long-term ACE inhibition doubles lifespan of hypertensive rats. Circulation. 1997;96:3164–3172. doi: 10.1161/01.cir.96.9.3164. [DOI] [PubMed] [Google Scholar]
- 236.Nevelsteen I, Van den Bergh A, Van der Mieren G, Vanderper A, Mubagwa K, Bult H, Herijgers P. NO-dependent endothelial dysfunction in type II diabetes is aggravated by dyslipidemia and hypertension, but can be restored by angiotensin-converting enzyme inhibition and weight loss. J Vasc Res. 2013;50:486–497. doi: 10.1159/000355221. [DOI] [PubMed] [Google Scholar]
- 237.de Cavanagh EM, Inserra F, Ferder L. Angiotensin II blockade: how its molecular targets may signal to mitochondria and slow aging. Coincidences with calorie restriction and mTOR inhibition. Am J Physiol Heart Circ Physiol. 2015;309:H15–H44. doi: 10.1152/ajpheart.00459.2014. [DOI] [PubMed] [Google Scholar]
- 238.Benigni A, Corna D, Zoja C, Sonzogni A, Latini R, Salio M, Conti S, Rottoli D, Longaretti L, Cassis P, Morigi M, Coffman TM, Remuzzi G. Disruption of the Ang II type 1 receptor promotes longevity in mice. J Clin Invest. 2009;119:524–530. doi: 10.1172/JCI36703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Linz W, Heitsch H, Scholkens BA, Wiemer G. Long-term angiotensin II type 1 receptor blockade with fonsartan doubles lifespan of hypertensive rats. Hypertension. 2000;35:908–913. doi: 10.1161/01.hyp.35.4.908. [DOI] [PubMed] [Google Scholar]
- 240.Spindler SR, Mote PL, Li R, Dhahbi JM, Yamakawa A, Flegal JM, Jeske DR, Li R, Lublin AL. beta1-Adrenergic receptor blockade extends the life span of Drosophila and long-lived mice. Age (Dordr) 2013;35:2099–2109. doi: 10.1007/s11357-012-9498-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Liao Y, Asakura M, Takashima S, Ogai A, Asano Y, Shintani Y, Minamino T, Asanuma H, Sanada S, Kim J, Kitamura S, Tomoike H, Hori M, Kitakaze M. Celiprolol, a vasodilatory beta-blocker, inhibits pressure overload-induced cardiac hypertrophy and prevents the transition to heart failure via nitric oxide-dependent mechanisms in mice. Circulation. 2004;110:692–699. doi: 10.1161/01.CIR.0000137831.08683.E1. [DOI] [PubMed] [Google Scholar]
- 242.Cleland JG, Teerlink JR, Senior R, et al. The effects of the cardiac myosin activator, omecamtiv mecarbil, on cardiac function in systolic heart failure: a double-blind, placebo-controlled, crossover, dose-ranging phase 2 trial. Lancet. 2011;378:676–683. doi: 10.1016/S0140-6736(11)61126-4. [DOI] [PubMed] [Google Scholar]
- 243.Shen YT, Malik FI, Zhao X, Depre C, Dhar SK, Abarzua P, Morgans DJ, Vatner SF. Improvement of cardiac function by a cardiac Myosin activator in conscious dogs with systolic heart failure. Circ Heart Fail. 2010;3:522–527. doi: 10.1161/CIRCHEARTFAILURE.109.930321. [DOI] [PubMed] [Google Scholar]
- 244.Teerlink JR, Clarke CP, Saikali KG, Lee JH, Chen MM, Escandon RD, Elliott L, Bee R, Habibzadeh MR, Goldman JH, Schiller NB, Malik FI, Wolff AA. Dose-dependent augmentation of cardiac systolic function with the selective cardiac myosin activator, omecamtiv mecarbil: a first-in-man study. Lancet. 2011;378:667–675. doi: 10.1016/S0140-6736(11)61219-1. [DOI] [PubMed] [Google Scholar]
- 245.Bakkehaug JP, Kildal AB, Engstad ET, Boardman N, Naesheim T, Ronning L, Aasum E, Larsen TS, Myrmel T, How OJ. Myosin Activator Omecamtiv Mecarbil Increases Myocardial Oxygen Consumption and Impairs Cardiac Efficiency Mediated by Resting Myosin ATPase Activity. Circ Heart Fail. 2015;8:766–775. doi: 10.1161/CIRCHEARTFAILURE.114.002152. [DOI] [PubMed] [Google Scholar]
- 246.Tarone G, Balligand JL, Bauersachs J, et al. Targeting myocardial remodelling to develop novel therapies for heart failure: a position paper from the Working Group on Myocardial Function of the European Society of Cardiology. Eur J Heart Fail. 2014;16:494–508. doi: 10.1002/ejhf.62. [DOI] [PubMed] [Google Scholar]
- 247.Loffredo FS, Nikolova AP, Pancoast JR, Lee RT. Heart failure with preserved ejection fraction: molecular pathways of the aging myocardium. Circ Res. 2014;115:97–107. doi: 10.1161/CIRCRESAHA.115.302929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Zile MR, Baicu CF, Ikonomidis JS, Stroud RE, Nietert PJ, Bradshaw AD, Slater R, Palmer BM, Van Buren P, Meyer M, Redfield MM, Bull DA, Granzier HL, LeWinter MM. Myocardial stiffness in patients with heart failure and a preserved ejection fraction: contributions of collagen and titin. Circulation. 2015;131:1247–1259. doi: 10.1161/CIRCULATIONAHA.114.013215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Hamdani N, Bishu KG, von Frieling-Salewsky M, Redfield MM, Linke WA. Deranged myofilament phosphorylation and function in experimental heart failure with preserved ejection fraction. Cardiovasc Res. 2013;97:464–471. doi: 10.1093/cvr/cvs353. [DOI] [PubMed] [Google Scholar]
- 250.Bishu K, Hamdani N, Mohammed SF, Kruger M, Ohtani T, Ogut O, Brozovich FV, Burnett JC, Jr, Linke WA, Redfield MM. Sildenafil and B-type natriuretic peptide acutely phosphorylate titin and improve diastolic distensibility in vivo. Circulation. 2011;124:2882–2891. doi: 10.1161/CIRCULATIONAHA.111.048520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Maraldi NM, Capanni C, Cenni V, Fini M, Lattanzi G. Laminopathies and lamin-associated signaling pathways. J Cell Biochem. 2011;112:979–992. doi: 10.1002/jcb.22992. [DOI] [PubMed] [Google Scholar]
- 252.Lattanzi G, Ortolani M, Columbaro M, Prencipe S, Mattioli E, Lanzarini C, Maraldi NM, Cenni V, Garagnani P, Salvioli S, Storci G, Bonafe M, Capanni C, Franceschi C. Lamins are rapamycin targets that impact human longevity: a study in centenarians. J Cell Sci. 2014;127:147–157. doi: 10.1242/jcs.133983. [DOI] [PubMed] [Google Scholar]
- 253.Baker DJ, Childs BG, Durik M, Wijers ME, Sieben CJ, Zhong J, Saltness RA, Jeganathan KB, Verzosa GC, Pezeshki A, Khazaie K, Miller JD, van Deursen JM. Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. Nature. 2016;530:184–189. doi: 10.1038/nature16932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Marfella R, Sasso FC, Cacciapuoti F, Portoghese M, Rizzo MR, Siniscalchi M, Carbonara O, Ferraraccio F, Torella M, Petrella A, Balestrieri ML, Stiuso P, Nappi G, Paolisso G. Tight glycemic control may increase regenerative potential of myocardium during acute infarction. J Clin Endocrinol Metab. 2012;97:933–942. doi: 10.1210/jc.2011-2037. [DOI] [PubMed] [Google Scholar]
- 255.Chiao YA, Ramirez TA, Zamilpa R, Okoronkwo SM, Dai Q, Zhang J, Jin YF, Lindsey ML. Matrix metalloproteinase-9 deletion attenuates myocardial fibrosis and diastolic dysfunction in ageing mice. Cardiovasc Res. 2012;96:444–455. doi: 10.1093/cvr/cvs275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Yabluchanskiy A, Ma Y, Chiao YA, Lopez EF, Voorhees AP, Toba H, Hall ME, Han HC, Lindsey ML, Jin YF. Cardiac aging is initiated by matrix metalloproteinase-9-mediated endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2014;306:H1398–H1407. doi: 10.1152/ajpheart.00090.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Han L, Li M, Liu Y, Han C, Ye P. Atorvastatin may delay cardiac aging by upregulating peroxisome proliferator-activated receptors in rats. Pharmacology. 2012;89:74–82. doi: 10.1159/000335783. [DOI] [PubMed] [Google Scholar]
- 258.Lee SJ, Won SY, Park SL, Song JH, Noh DH, Kim HM, Yin CS, Kim WJ, Moon SK. Rosa hybrida extract suppresses vascular smooth muscle cell responses by the targeting of signaling pathways, cell cycle regulation and matrix metalloproteinase-9 expression. Int J Mol Med. 2016;37:1119–1126. doi: 10.3892/ijmm.2016.2504. [DOI] [PubMed] [Google Scholar]
- 259.Chen ZZ, Yang DD, Zhao Z, Yan H, Ji J, Sun XL. Memantine mediates neuroprotection via regulating neurovascular unit in a mouse model of focal cerebral ischemia. Life Sci. 2016;6:21719. doi: 10.1016/j.lfs.2016.02.081. [DOI] [PubMed] [Google Scholar]
- 260.Takai S, Jin D. Improvement of cardiovascular remodeling by chymase inhibitor. Clin Exp Pharmacol Physiol. 2016;43:387–393. doi: 10.1111/1440-1681.12549. [DOI] [PubMed] [Google Scholar]
- 261.Katsimpardi L, Litterman NK, Schein PA, Miller CM, Loffredo FS, Wojtkiewicz GR, Chen JW, Lee RT, Wagers AJ, Rubin LL. Vascular and neurogenic rejuvenation of the aging mouse brain by young systemic factors. Science. 2014;344:630–634. doi: 10.1126/science.1251141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Zhu Y, Tchkonia T, Pirtskhalava T, et al. The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell. 2015;14:644–658. doi: 10.1111/acel.12344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Zhu Y, Tchkonia T, Fuhrmann-Stroissnigg H, Dai HM, Ling YY, Stout MB, Pirtskhalava T, Giorgadze N, Johnson KO, Giles CB, Wren JD, Niedernhofer LJ, Robbins PD, Kirkland JL. Identification of a Novel Senolytic Agent, Navitoclax, Targeting the Bcl-2 Family of Anti-Apoptotic Factors. Aging Cell. 2015 doi: 10.1111/acel.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.