Synopsis
Human cardiomyocytes express three distinct types of delayed rectifier potassium channels. hERG1 channels conduct the rapidly activating current IKr, KCNQ1/KCNE1 channels conduct the slowly activating current IKs, and Kv1.5 channels conduct an ultrarapid activating current IKur. Here we provide a general overview of the mechanistic and structural basis of ion selectivity, gating and pharmacology of the three types of cardiac delayed rectifier K+ channels. Most blockers bind to S6 residues that line the central cavity of the channel, whereas activators interact with the channel at four symmetrical binding sites outside the cavity.
Keywords: gating, hERG, KCNA5, KCNE1, KCNQ1, pharmacology, potassium channel
Introduction
In cardiomyocytes, multiple types of outward delayed rectifier K+ current (IK) mediate the late repolarization phase of action potentials 1. In human cardiomyocytes, three distinct types of IK are recognized. A rapidly activating current (IKr) is conducted by channels formed by coassembly of human ether-a-go-go-related gene, hERG1 (Kv11.1; gene: KCNH2) subunits, both full-length hERG1a α-subunits 2,3 and alternatively spliced hERG1b α-subunits 4. A slowly activating current (IKs) is conducted by channels formed by coassembly of KCNQ1 (Kv7.1; gene: KCNQ1) α-subunits and auxiliary KCNE1 β-subunits 5,6. In human atrial, but not ventricular, myocytes an ultrarapid activating current (IKur) is also present and conducted by channels formed by Kv1.5 (gene: KCN5A) α-subunits 7,8. Identification of the molecular basis of IKur, IKr and IKs combined with patch clamp studies of heterologously expressed channels spawned copious biophysical studies designed to probe the molecular mechanisms of channel gating and enabled the screening of small molecule libraries to discover novel compounds that either inhibit or activate these channels. Detailed descriptions of the molecular basis of Kv1.5, hERG1 and KCNQ1 channel gating are reviewed elsewhere 9–12. Here we provide a general overview of the structural basis of channel gating and feature recent findings regarding modulation of cardiac delayed rectifier K+ channel by low molecular weight compounds and peptides.
Structural basis of voltage-dependent potassium channel gating
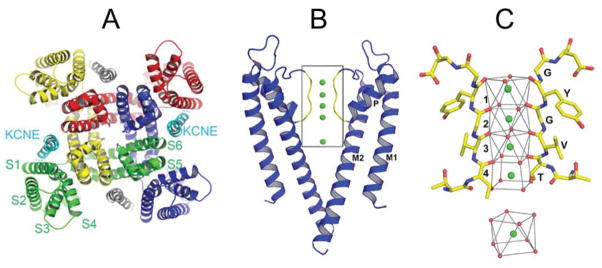
Insights into the molecular mechanisms of K+ channel gating have been provided by early biophysical studies of Drosophila Shaker and other voltage-gated K+ (Kv) channels combined with structural biology studies of several bacterial channels (KcsA, MthK, KvAP) and vertebrate Kv1.2 channels. Similar to other Kv channel subunits, hERG1, KCNQ1 and Kv1.5 subunits each contain 6 transmembrane α-helical segments (S1–S6) and functional channels are formed by coassembly of four identical or highly similar α-subunits into a tetrameric complex (Fig. 1A). In each subunit, segments S1–S4 form a voltage sensing domain (VSD) and segments S5 and S6 contribute to the central pore-forming domain. The S6 segments line the central cavity of the channel and the cytoplasmic ends of each segment criss-cross one another (the “S6 bundle crossing”) to form a narrow aperture (the activation gate) when the channel is in a closed state. The S4 segment contains multiple basic (positively charged) amino acids and thus serves as the primary voltage sensing structure. In response to membrane depolarization, the S4 segments move in an outward direction and the attached cytoplasmic S4–S5 linker acts as an electromechanical coupler to link the S4 movement to opening of the activation gate (i.e., an outward splaying of the S6 bundle crossing opening) 13. When the activation gate is in an open configuration, hydrated K+ ions within the cytoplasm diffuse into the central cavity in response to the outwardly directed electrochemical driving force. As K+ ions enter the selectivity filter (Fig. 1B), they are stripped of their surrounding water molecules and move stepwise from one high affinity binding site to another until they reach the extracellular vestibule where they are again rehydrated. The selectivity filter of K+-selective channels is a narrow lumen that is lined by five residues (TVGYG) contributed by each of the four subunits. The hydroxyl group from the Thr residue and the backbone carbonyl oxygen atoms of four following amino acids together form an oxygen network that coordinate dehydrated K+ ions in a manner that closely resembles the oxygen atoms from 8 water molecules that form the hydration shell surrounding the ion in solution 14.
Fig. 1.
Structural features of voltage-gated K+ channels. A, Functional channels are composed of four identical or highly related α-subunits. The S1–S4 transmembrane segments form the VSD. The S5 and S6 segments form the pore domain. In IKs channels, four KCNQ1 α-subunits are joined by two (or more) KCNE1 β-subunits that are positioned in the cleft as indicated. From Nakajo K. and Kubo Y. KCNQ1 channel modulation by KCNE proteins via the voltage-sensing domain. J Physiol. 2015;593:2617–2625. B, side-view of the pore domain of the KcsA bacterial K+ channel in a closed state. Only two of the four subunits are shown; selectivity filter is boxed. c, the selectivity filter of a K+ channel (KcsA) is a narrow lumen where dehydrated K+ ions (green sphere) transiently bind to sites (1–4) formed by oxygen atoms contributed by TYGYG residues that form the structure. Bottom panel shows a single K+ ion surrounded by 8 water molecules. B and C: From Alam A. and Youxing J. Structural studies of ion selectivity in tetrameric cation channels. J Gen Physiol. 2011;137(5):397–403; with permission.
Activation gating
The amino acid sequence of the Kv1.5 channel is highly homologous to Kv1.2 and so it is reasonable to assume the structural basis of gating described for Kv1.2 also apply to cardiac Kv1.5 channels. Some of the details differ for hERG1 and KCNQ1/KCNE1 channels. Recently it was reported that physical continuity between the S4 segment and pore domain is not required for normal voltage dependence of activation (and inactivation) gating in hERG1 channels. Channels could still gate relatively normally when subunits were split into two pieces at the S4–S5 linker, a finding inconsistent with the simple idea of electromechanical coupling 15. For hERG1 channels, specific interactions between Asp540 in the S4–S5 linker and Leu666 in the S6 segment are a key component of activation gating 16,17. Presumably these interactions can still occur in a split channel. In the heart, the biophysical properties of KCNQ1 channels are modified by KCNE1 β-subunits. Channels formed by coassembly of KCNE1 subunits (2 or more per channel) to KCNQ1 homotetramers exhibit an increased single channel conductance, open at more positive potentials and have a slower rate of activation. Voltage sensor movement associated with KCNQ1 channel activation is divided into two steps with distinct voltage dependences and kinetics 18, corresponding to an intermediate-open or a high permeation activated-open state 19. KCNE1 subunits inhibit the intermediate-open state and facilitate the activated-open states by altering the interactions between the VSDs and the pore domain 19. The binding of intracellular phosphatidylinositol 4,5-bisphosphate (PIP2) or ATP promote coupling between voltage sensing at S4 segments and the pore domain in KCNQ1/KCNE1 channels 20,21.
Inactivation gating
Kv1.5 channels exhibit a very slow and time-independent C-type inactivation at depolarized potentials, a process that involves cooperative subunit interactions in related Kv1 channels 22,23. Auxiliary Kvβ1.2 and Kvβ1.3 subunits interact with the C-terminal domain of Kv1.5 24 to induce rapid inactivation 25. It is unclear to what extent Kvβ subunits alter Kv1.5 channel gating in human atrial myocytes. KCNQ1 channels inactivate slightly at positive potentials, but KCNQ1/KCNE1 channels do not inactivate 26. In hERG1, C-type inactivation is extremely fast and voltage-dependent 27,28 and occurs in sequential steps that culminate in a subtle change in the conformation of the selectivity filter 29. We employed concatenated tetramers to demonstrate that the extent of subunit cooperativity was dependent on the location of the mutations employed to probe inactivation and that the final step in the gating process was mediated by a concerted, all-or-none cooperative subunit interaction 30.
Deactivation
Return of the transmembrane potential to a negative level (as occurs during repolarization of an action potential) induces channels transition from an open to a closed state, a process called deactivation. The slow rate of deactivation of hERG1 channels is dependent on multiple interactions between the cytoplasmic N-terminus of one subunit with the cytoplasmic C-terminus of an adjacent subunit 31–33. Slow deactivation is a fully cooperative process (i.e., all-or-none), requiring the N-C interaction between all four subunits. Disruption of a single N-C interaction is sufficient to greatly accelerate deactivation 34. This has physiological significance as hERG1 channels can be formed by coassembly of hERG1a subunits that contain a full-length N-terminus and hERG1b subunits that have a truncated N-terminus 4,35 that cannot interact with the C-terminus to slow deactivation.
Molecular pharmacology of cardiac delayed rectifier K+ channels
Inhibitors
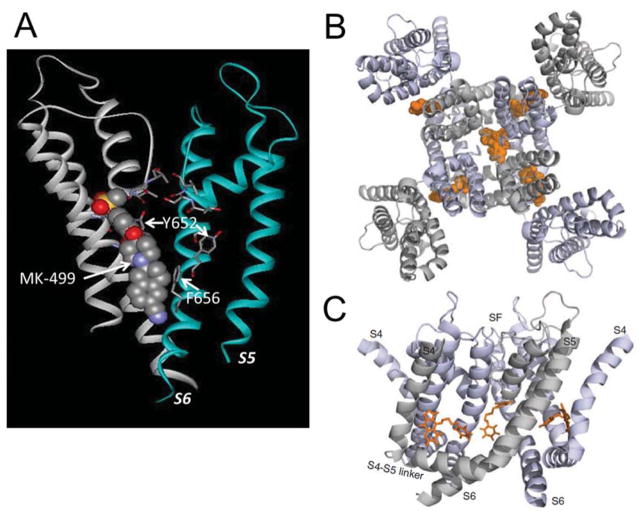
The majority of Kv1.5 inhibitors are pore blockers. Several potent open channel blockers of Kv1.5, including AVE0118, S0100176, vernakalant, DPO-1, MK-0448, anandamide and acacetin, were developed to selectively block IKur in atrium as a potential therapy for atrial fibrillation (AF) 36–42. These Kv1.5 blockers share an overlapping binding site, including 5 critical residues that face towards the lumen of the central cavity: Thr-479, Thr-480, Val-505, Ile-508, and Val-512 39,43–46. More recently, Marzian et al. identified Psora-4 as a unique Kv1.5 inhibitor that is both a pore blocker and an allosteric gating modifier 47. Besides direct pore blockage, Psora-4 also binds to four symmetrical side pockets distant from the central cavity (Fig. 2A). The amino acids that form the pore of Kv1 channels are highly conserved, challenging efforts to develop specific Kv1.5 blockers. Allosteric gating inhibitors such as Psora-4 offer an alternative approach to the design of channel-specific drugs.
Fig. 2.
K+ channel inhibitors. A, Model of the hERG1 channel pore domain showing block of the central cavity by MK-499, a class III antiarrhythmic agent. The S5 and S6 segments for two of the four subunits are shown. The key binding residues in the S6 segment (Y652 and F656) are indicated. From Mitcheson JS, Chen J, Lin M, Culberson C, and Sanguinetti MC A structural basis for drug-induced long QT syndrome. Proc Natl Acad Sci USA. 2000;97:12329–12333. B and C, Psora-4 (orange) binds to multiple sites on the Kv1.5 channel viewed from the extracellular space (B) or from the side (C). From Marzian S, Stansfeld PJ, Rapedius M, et al. Side pockets provide the basis for a new mechanism of Kv channel-specific inhibition. Nat Chem Biol. 2013;9:507–513; with permission.
The primary motivation driving the discovery of Kv1.5 blockers has been its predicted usefulness for pharmacological cardioversion of recent onset AF or prevention of AF episodes by a mechanism that would not affect the electrophysiological properties of the ventricles. Specifically, because Kv1.5 is highly expressed in human atria but not in the ventricles, block of these channels would be expected to specifically prolong the APD of atrial myocytes (and atrial effective refractory period, AERP) without risk of inducing QT prolongation. The clinical experience to date has not been encouraging for this mechanism. Although MK-0448 was shown in preclinical studies to prolong AERP without effects on the ventricle, the compound was without effect on AERP in healthy human subjects. This finding was predicted by previous mathematical modeling of human atrial action potentials. Simulated inhibition of IKur was shown to increase plateau height, leading to additional activation of IKr and no net change in APD 48. However, as noted by Ravens et al 49 MK-0448 was evaluated by electrophysiological testing over a frequency range lower than what is typically observed during AF. It remains to be demonstrated that Kv1.5 channel inhibition will provide protection against AF in relevant patient populations.
hERG1 channels are blocked by a wide spectrum of compounds, including quinidine, d-sotalol and many other “class III” antiarrhythmic agents such as the highly potent methanesulfonanilides dofetilide and ibutilide. All of these compounds block hERG1 channels by preferentially plugging the central cavity of the channel (Fig. 2B, C). In addition to these antiarrhythmic drugs, several other commonly used medications with diverse chemical structures (e.g., cisapride, terfenadine, astemizole, mexifloxacin, and tamoxifen) were shown to cause high-affinity block on hERG1/IKr channels and hence, prolong the QT interval 50. Many hERG1 blockers have an unacceptable risk of inducing arrhythmia. Unintended drug-induced QT prolongation with the potential to cause acquired long QT Syndrome (LQTS) increases the risk of ventricular tachyarrhythmia (most commonly, torsade de pointes) in patients and hence has prompted the withdrawal or restriction of these marketed drugs. The structural basis for high-affinity binding of hERG1/IKr channel blockers was revealed using a site-directed mutagenesis approach. Two aromatic residues (i.e., Y652 and F656) located in all four S6 segments that line the central cavity of the channel are the most critical determinants for drug interaction 51,52. Inactivation-deficient hERG1 mutant channels (e.g., S620T, S631A, G628C/S631C) are much less sensitive to block by these compounds 53–56, so it was commonly assumed that drugs can only bind with high affinity to the inactivated state of the channel. However, a detailed analysis of concatenated hERG1 tetramers containing a variable number of S620T, G628C/S631C or S631A mutation subunits indicate instead that these mutant subunits allosterically interrupt drug binding in a manner that is independent of their ability to disrupt inactivation gating 57. Peptide toxins isolated from scorpion venoms, such as ErgTx1 and BeKm-1, bind to the external region of the pore to block channels with nanomolar potency 58,59. Other peptide toxins act as allosteric modifiers of activation gating, including APETx1 from sea anemone and several tarantula toxins. These peptides directly interact with the VSD of hERG1 and reduce current magnitude by shifting the voltage dependence of activation to more positive potentials 60,61.
Chromanol 293B is a relatively selective IKs blocker which was initially developed as a class III antiarrhythmic drug to prolong action potential duration 62,63. The benzodiazepine L-7 is a more potent blocker that binds to specific residues in the S6 segments of KCNQ1 channels 64,65. Other compounds such as indapamide, propofol, and thiopentone exert lower affinity block of KCNQ1 channels 66,67. KCNE1 subunits displayed allosteric action to facilitate the block of chromanol 293B to KCNQ1 channels with a 6–100 fold increased binding affinity 68.
The amino acids in the S6 segments that line the central cavity of K+ channels are often highly conserved amongst members of closely related channels (e.g., Kv1.1, 1.2, 1.3, 1.4 and 1.5). This has been a major impediment to the discovery of pore blockers that are highly channel-specific. Another approach to inhibiting K+ flux is to modulate channel gating processes (e.g., shift the voltage dependence of activation to more positive potentials) with compounds that bind to structural elements (e.g., voltage-sensor domain) that are not as highly conserved as the residues in the S6 segment that form the channel pore. We anticipate that this approach will be greatly facilitated as more K+ channel structures are elucidated.
Activators
Congenital LQTS is a disorder of cardiac repolarization that predisposes affected individuals to an increased risk of cardiac arrhythmia and sudden cardiac death. The most common cause of LQTSs are loss of function mutations in either hERG1 or KCNQ1 channels, resulting in a reduction of IKr and IKs, and a prolongation of ventricular repolarization easily quantified as a longer QTc interval 69–71. Thus, compounds that activate hERG1 or KCNQ1 channels are an obvious potential approach to restoring normal repolarization for the vast majority of congenital LQTS patients.
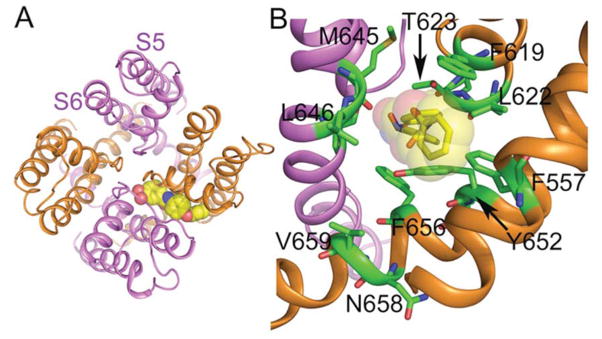
In the past 10 years, routine safety screening of compounds for undesirable hERG1 activity led to the serendipitous discovery of several hERG1 activators that act by different mechanisms to allosterically modify channel gating. These agents can either slow deactivation, attenuate C-type inactivation, enhance single channel open probability, induce a hyperpolarizing shift in the voltage dependence of activation, or exert a combination of two or more of these actions 72. RPR-260243 was the first reported synthetic hERG1 activator. This compound markedly slows the rate of hERG1 channel deactivation and causes a mild attenuation of inactivation with no effect on the voltage dependence of activation 73,74. Ginsenoside Rg3, a natural product isolated from ginseng root also dramatically slows hERG1 deactivation rate, but it also causes a modest negative shift in the voltage dependence of activation 75. ICA-105574 76,77, ML-T531 78 and AZSMO-23 79 profoundly enhance outward hERG1 current by greatly attenuating C-type inactivation, and modestly slow deactivation without affecting the voltage dependence of channel activation. PD-118057 enhances hERG1 current by increasing channel open probability and causing a positive shift in the voltage dependence of inactivation 80. The putative binding sites for RPR-260243, ICA-105574, and PD-118057 were revealed by the combined approach of alanine-scanning mutagenesis and molecular modeling. Although the specific residues of hERG1 that interact with these agonists vary, all three compounds interact with the channel via a hydrophobic pocket located between two adjacent subunits of the pore domain (Fig. 3) 74,77,80 such that a homotetrameric hERG1 channel would contain four such identical binding sites. Analysis of concatenated hERG1 tetramers revealed that all four binding sites and cooperative subunit interactions are required to achieve maximal effects by ICA-105574 or PD-118057 81. Maximal slowing of deactivation by RPR-260243 requires all four hERG1 subunits, whereas only two or more subunits were sufficient to achieve a maximal effect on inactivation 82. In addition to ginsenoside Rg3 75, mallotoxin 83 and KB130015 84 also shift the voltage dependence of hERG1 activation to more negative potentials; however, the binding site for these agents has not yet been defined. NS1643 shifts the voltage dependence of activation to more negative potentials and of inactivation to more positive potentials, but effects vary depending upon the heterologous expression system employed 85–87. The binding site for NS1643 has eluded definitive identification, but based on detailed molecular modeling and analysis of L529I hERG1 mutant channels, it is likely that NS1643 interacts indirectly with the VSD to facilitate opening of hERG1 channels 88.
Fig. 3.
Binding site for ICA-105574, a hERG1 channel activator. A, open-state pore domain of the channel as viewed from the extracellular space. ICA is shown in space fill occupying one of the four symmetrical sites available. VSDs are not shown. B, side view of the binding site with important binding sites residues indicated in single letter code. From Garg V, Stary-Weinzinger A, Sachse F, Sanguinetti, MC. Molecular determinants for activation of human ether-a-go-go-related gene 1 potassium channels by 3-nitro-n-(4-phenoxyphenyl) benzamide. Mol Pharmacol. 2011;80:630–637; with permission.
R-L3 was the first IKs activator to be described 89,90 and was shown to slow the rate of deactivation and cause a hyperpolarizing shift in the voltage dependence of channel activation. Overexpression of KCNE1 subunits with KCNQ1 channels abolished the action of R-L3, suggesting that the single transmembrane domain KCNE1 subunit either competes directly, or allosterically, with R-L3 for binding to KCNQ1 channel subunits. The putative binding site for R-L3 between S5 and S6 segments 90 strongly suggests that it too will bind with four-fold symmetry as described for the hERG1 activators. Zinc pyrithione (ZnPy) is a potent activator of KCNQ2 and KCNQ3 channels, but also activates KCNQ1 91,92. ZnPy attenuates inactivation, slows the rate of activation and deactivation of KCNQ1 channels and like R-L3 its actions are abolished by over-expression of KCNE1 subunits 91. Most recently, high-throughput screening identified the compound ML277 as a potent KCNQ1 agonist 93,94 whose efficacy is also reduced by KCNE1 95. A combined approach of electrophysiology and molecular dynamic simulation indicated that ML277 binds to the side pockets between adjacent KCNQ1 subunits to allosterically modify channel gating 94. Nonspecific compounds such as the chloride channel blockers mefenamic acid and DIDS, and the anti-bacterial hexachlorophene can also activate IKs, but these compounds exert stronger potentiation on KCNQ1/KCNE1 channels than on homomeric KCNQ1 channels 96,97. Unlike other KCNQ1 channel activators, the negative shift of voltage dependence of channel activation produced by phenylboronic acid is independent of KCNE1 subunits 98. Polyunsaturated fatty acids (PUFAs) are the most recently identified KCNQ1 agonists 99. Electrostatic interactions between the negatively charged head group of PUFAs, such as docosahexaenoic acid, and the basic residues in the S4 segment of KCNQ1 shift the voltage dependence of channel activation to more negative potentials (maximal shift of -15 mV) at concentrations similar to total circulating plasma levels of PUFAs. Interestingly, KCNE1 subunits abolish the agonist effect by promoting PUFA protonation, indicating that natural PUFAs do not affect IKs channels in the heart 99. If excessive shortening of QTc can be avoided, hERG1 or KCNQ1/KCNE1 activators may be useful for prevention of arrhythmia associated with prolonged ventricular repolarization in LQTS patients. They might be particularly useful for LQTS patients with particularly aggressive arrhythmia syndromes, who enter periods of arrhythmic storm refractory to conventional management with repeated defibrillation shocks and increased mortality risk.
Summary
Fueled by the molecular cloning of genes encoding the many channels that conduct the wide diversity of known ionic currents, the last two decades have witnessed a great leap forward in our understanding of the structural and mechanistic basis of potassium channel function. Due to their well-recognized importance in cardiac repolarization and the severe medical consequence of inherited gene mutations, the pharmacology of cardiac delayed rectifier K+ channels has been explored extensively. In the past few decades, high throughput drug screening efforts have focused on the discovery of specific channel blockers to treat atrial fibrillation (Kv1.5 channel blockers) or ventricular arrhythmia (hERG1 and KCNQ1/KCNE1 blockers). More recently, hERG1 and KCNQ1 channel activators were discovered that may prove useful for treatment of ventricular arrhythmia associated with prolonged QTc intervals. It is anticipated that future antiarrhythmic drug discovery efforts will rely less on screening of large compound libraries and instead capitalize on our increasingly sophisticated understanding of channel structure and gating mechanisms to enable a rational, knowledge-based drug design approach.
Key Points.
Although the structures of cardiac delayed rectifier K+ channels, including Kv1.5, Kv7.1 (hERG1) and Kv11.1 (KCNQ1) have not been determined, the molecular basis of their function can be inferred from biophysical studies and known structural features of other K+ channels.
The molecular basis of action for several blockers and activators of cardiac delayed rectifier K+ channels have been determined primarily by functional analysis of mutant channels that alter drug affinity.
Most blockers inhibit K+ conductance by binding to the central cavity of the channel. In contrast, hERG1 and KCNQ1 activators bind outside the cavity to four symmetrical sites and alter one or more gating properties.
Footnotes
Disclosure Statement: The authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Noble D, Tsien RW. Outward membrane currents activated in the plateau range of potentials in cardiac Purkinje fibres. J Physiol. 1969;200:205–231. doi: 10.1113/jphysiol.1969.sp008689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sanguinetti MC, Jiang C, Curran ME, Keating MT. A mechanistic link between an inherited and an acquired cardiac arrhythmia: HERG encodes the IKr potassium channel. Cell. 1995;81:299–307. doi: 10.1016/0092-8674(95)90340-2. [DOI] [PubMed] [Google Scholar]
- 3.Trudeau MC, Warmke JW, Ganetzky B, Robertson GA. HERG, a human inward rectifier in the voltage-gated potassium channel family. Science. 1995;269:92–95. doi: 10.1126/science.7604285. [DOI] [PubMed] [Google Scholar]
- 4.Jones EM, Roti Roti EC, Wang J, Delfosse SA, Robertson GA. Cardiac IKr channels minimally comprise hERG 1a and 1b subunits. J Biol Chem. 2004;279:44690–44694. doi: 10.1074/jbc.M408344200. [DOI] [PubMed] [Google Scholar]
- 5.Sanguinetti MC, Curran ME, Zou A, et al. Coassembly of KVLQT1 and minK (IsK) proteins to form cardiac IKs potassium channel. Nature. 1996;384:80–83. doi: 10.1038/384080a0. [DOI] [PubMed] [Google Scholar]
- 6.Barhanin J, Lesage F, Guillemare E, et al. KVLQT1 and lsK (minK) proteins associate to form the IKs cardiac potassium current. Nature. 1996;384:78–80. doi: 10.1038/384078a0. [DOI] [PubMed] [Google Scholar]
- 7.Li GR, Feng J, Wang Z, Fermini B, Nattel S. Adrenergic modulation of ultrarapid delayed rectifier K+ current in human atrial myocytes. Circ Res. 1996;78:903–915. doi: 10.1161/01.res.78.5.903. [DOI] [PubMed] [Google Scholar]
- 8.Wang Z, Fermini B, Nattel S. Sustained depolarization-induced outward current in human atrial myocytes. Evidence for a novel delayed rectifier K+ current similar to Kv1.5 cloned channel currents. Circ Res. 1993;73:1061–1076. doi: 10.1161/01.res.73.6.1061. [DOI] [PubMed] [Google Scholar]
- 9.Vandenberg JI, Perry MD, Perrin MJ, et al. hERG K+ channels: structure, function, and clinical significance. Physiol Rev. 2012;92:1393–1478. doi: 10.1152/physrev.00036.2011. [DOI] [PubMed] [Google Scholar]
- 10.Nakajo K, Kubo Y. KCNQ1 channel modulation by KCNE proteins via the voltage-sensing domain. J Physiol. 2015;593:2617–2625. doi: 10.1113/jphysiol.2014.287672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liin SI, Barro-Soria R, Larsson HP. The KCNQ1 channel - remarkable flexibility in gating allows for functional versatility. J Physiol. 2015;593:2605–2615. doi: 10.1113/jphysiol.2014.287607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmitt N, Grunnet M, Olesen SP. Cardiac potassium channel subtypes: new roles in repolarization and arrhythmia. Physiol Rev. 2014;94:609–653. doi: 10.1152/physrev.00022.2013. [DOI] [PubMed] [Google Scholar]
- 13.Long SB, Campbell EB, Mackinnon R. Voltage sensor of Kv1.2: structural basis of electromechanical coupling. Science. 2005;309:903–908. doi: 10.1126/science.1116270. [DOI] [PubMed] [Google Scholar]
- 14.Zhou Y, Morais-Cabral JH, Kaufman A, MacKinnon R. Chemistry of ion coordination and hydration revealed by a K+ channel-Fab complex at 2.0 A resolution. Nature. 2001;414:43–48. doi: 10.1038/35102009. [DOI] [PubMed] [Google Scholar]
- 15.Lorinczi E, Gomez-Posada JC, de la Pena P, et al. Voltage-dependent gating of KCNH potassium channels lacking a covalent link between voltage-sensing and pore domains. Nat Commun. 2015;6:6672. doi: 10.1038/ncomms7672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferrer T, Rupp J, Piper DR, Tristani-Firouzi M. The S4–S5 linker directly couples voltage sensor movement to the activation gate in the human ether-a’-go-go-related gene (hERG) K+ channel. J Biol Chem. 2006;281:12858–12864. doi: 10.1074/jbc.M513518200. [DOI] [PubMed] [Google Scholar]
- 17.Tristani-Firouzi M, Chen J, Sanguinetti MC. Interactions between S4–S5 linker and S6 transmembrane domain modulate gating of HERG K+ channels. J Biol Chem. 2002;277:18994–19000. doi: 10.1074/jbc.M200410200. [DOI] [PubMed] [Google Scholar]
- 18.Barro-Soria R, Rebolledo S, Liin SI, et al. KCNE1 divides the voltage sensor movement in KCNQ1/KCNE1 channels into two steps. Nat Commun. 2014;5:3750. doi: 10.1038/ncomms4750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zaydman MA, Kasimova MA, McFarland K, et al. Domain-domain interactions determine the gating, permeation, pharmacology, and subunit modulation of the IKs ion channel. Elife. 2014;3:e03606. doi: 10.7554/eLife.03606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zaydman MA, Silva JR, Delaloye K, et al. Kv7.1 ion channels require a lipid to couple voltage sensing to pore opening. Proc Natl Acad Sci USA. 2013;110:13180–13185. doi: 10.1073/pnas.1305167110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Y, Gao J, Lu Z, et al. Intracellular ATP binding is required to activate the slowly activating K+ channel IKs. Proc Natl Acad Sci USA. 2013;110:18922–18927. doi: 10.1073/pnas.1315649110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ogielska EM, Zagotta WN, Hoshi T, et al. Cooperative subunit interactions in C-type inactivation of K channels. Biophys J. 1995;69:2449–2457. doi: 10.1016/S0006-3495(95)80114-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Panyi G, Sheng Z, Deutsch C. C-type inactivation of a voltage-gated K+ channel occurs by a cooperative mechanism. Biophys J. 1995;69:896–903. doi: 10.1016/S0006-3495(95)79963-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tipparaju SM, Li XP, Kilfoil PJ, et al. Interactions between the C-terminus of Kv1.5 and Kvbeta regulate pyridine nucleotide-dependent changes in channel gating. Pflugers Arch. 2012;463:799–818. doi: 10.1007/s00424-012-1093-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heinemann SH, Rettig J, Graack HR, Pongs O. Functional characterization of Kv channel beta-subunits from rat brain. J Physiol. 1996;493:625–633. doi: 10.1113/jphysiol.1996.sp021409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tristani-Firouzi M, Sanguinetti MC. Voltage-dependent inactivation of the human K+ channel KvLQT1 is eliminated by association with minimal K+ channel (minK) subunits. J Physiol. 1998;510:37–45. doi: 10.1111/j.1469-7793.1998.037bz.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spector PS, Curran ME, Zou A, Keating MT, Sanguinetti MC. Fast inactivation causes rectification of the IKr channel. J Gen Physiol. 1996;107:611–619. doi: 10.1085/jgp.107.5.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith PL, Baukrowitz T, Yellen G. The inward rectification mechanism of the HERG cardiac potassium channel. Nature. 1996;379:833–836. doi: 10.1038/379833a0. [DOI] [PubMed] [Google Scholar]
- 29.Wang DT, Hill AP, Mann SA, Tan PS, Vandenberg JI. Mapping the sequence of conformational changes underlying selectivity filter gating in the KV11.1 potassium channel. Nat Struct Mol Biol. 2011;18:35–41. doi: 10.1038/nsmb.1966. [DOI] [PubMed] [Google Scholar]
- 30.Wu W, Gardner A, Sanguinetti MC. Cooperative subunit interactions mediate fast C-type inactivation of hERG1 K+ channels. J Physiol. 2014;592:4465–4480. doi: 10.1113/jphysiol.2014.277483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ng CA, Phan K, Hill AP, Vandenberg JI, Perry MD. Multiple interactions between cytoplasmic domains regulate slow deactivation of Kv11.1 channels. J Biol Chem. 2014;289:25822–25832. doi: 10.1074/jbc.M114.558379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gianulis EC, Liu Q, Trudeau MC. Direct interaction of eag domains and cyclic nucleotide-binding homology domains regulate deactivation gating in hERG channels. J Gen Physiol. 2013;142:351–366. doi: 10.1085/jgp.201310995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gustina AS, Trudeau MC. hERG potassium channel gating is mediated by N- and C-terminal region interactions. J Gen Physiol. 2011;137:315–325. doi: 10.1085/jgp.201010582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thomson SJ, Hansen A, Sanguinetti MC. Concerted all-or-none subunit interactions mediate slow deactivation of human ether-a-go-go-related gene K+ channels. J Biol Chem. 2014;289:23428–23436. doi: 10.1074/jbc.M114.582437. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 35.Jones DK, Liu F, Vaidyanathan R, et al. hERG 1b is critical for human cardiac repolarization. Proc Natl Acad Sci USA. 2014;111:18073–18077. doi: 10.1073/pnas.1414945111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Friederich P, Pfizenmayer H. The novel Kv1.5 channel blocker vernakalant for successful treatment of new-onset atrial fibrillation in a critically ill abdominal surgical patient. Br J Anaesth. 2011;107:644–645. doi: 10.1093/bja/aer278. [DOI] [PubMed] [Google Scholar]
- 37.Li GR, Wang HB, Qin GW, et al. Acacetin, a natural flavone, selectively inhibits human atrial repolarization potassium currents and prevents atrial fibrillation in dogs. Circulation. 2008;117:2449–2457. doi: 10.1161/CIRCULATIONAHA.108.769554. [DOI] [PubMed] [Google Scholar]
- 38.Knobloch K, Brendel J, Rosenstein B, et al. Atrial-selective antiarrhythmic actions of novel IKur vs. IKr, IKs, and IKAch class Ic drugs and beta blockers in pigs. Med Sci Monit. 2004;10:BR221–228. [PubMed] [Google Scholar]
- 39.Decher N, Pirard B, Bundis F, et al. Molecular basis for Kv1.5 channel block: conservation of drug binding sites among voltage-gated K+ channels. J Biol Chem. 2004;279:394–400. doi: 10.1074/jbc.M307411200. [DOI] [PubMed] [Google Scholar]
- 40.Blaauw Y, Gogelein H, Tieleman RG, et al. “Early” class III drugs for the treatment of atrial fibrillation: efficacy and atrial selectivity of AVE0118 in remodeled atria of the goat. Circulation. 2004;110:1717–1724. doi: 10.1161/01.CIR.0000143050.22291.2E. [DOI] [PubMed] [Google Scholar]
- 41.Lagrutta A, Wang J, Fermini B, Salata JJ. Novel, potent inhibitors of human Kv1.5 K+ channels and ultrarapidly activating delayed rectifier potassium current. J Pharmacol Exp Ther. 2006;317:1054–1063. doi: 10.1124/jpet.106.101162. [DOI] [PubMed] [Google Scholar]
- 42.Barana A, Amoros I, Caballero R, et al. Endocannabinoids and cannabinoid analogues block cardiac hKv1.5 channels in a cannabinoid receptor-independent manner. Cardiovasc Res. 2010;85:56–67. doi: 10.1093/cvr/cvp284. [DOI] [PubMed] [Google Scholar]
- 43.Eldstrom J, Wang Z, Xu H, et al. The molecular basis of high-affinity binding of the antiarrhythmic compound vernakalant (RSD1235) to Kv1.5 channels. Mol Pharmacol. 2007;72:1522–1534. doi: 10.1124/mol.107.039388. [DOI] [PubMed] [Google Scholar]
- 44.Decher N, Kumar P, Gonzalez T, Pirard B, Sanguinetti MC. Binding site of a novel Kv1.5 blocker: a “foot in the door” against atrial fibrillation. Mol Pharmacol. 2006;70:1204–1211. doi: 10.1124/mol.106.026203. [DOI] [PubMed] [Google Scholar]
- 45.Wu HJ, Wu W, Sun HY, et al. Acacetin causes a frequency- and use-dependent blockade of hKv1.5 channels by binding to the S6 domain. J Mol Cell Cardiol. 2011;51:966–973. doi: 10.1016/j.yjmcc.2011.08.022. [DOI] [PubMed] [Google Scholar]
- 46.Du YM, Zhang XX, Tu DN, et al. Molecular determinants of Kv1.5 channel block by diphenyl phosphine oxide-1. J Mol Cell Cardiol. 2010;48:1111–1120. doi: 10.1016/j.yjmcc.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 47.Marzian S, Stansfeld PJ, Rapedius M, et al. Side pockets provide the basis for a new mechanism of Kv channel-specific inhibition. Nat Chem Biol. 2013;9:507–513. doi: 10.1038/nchembio.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Courtemanche M, Ramirez RJ, Nattel S. Ionic targets for drug therapy and atrial fibrillation-induced electrical remodeling: insights from a mathematical model. Cardiovasc Res. 1999;42:477–489. doi: 10.1016/s0008-6363(99)00034-6. [DOI] [PubMed] [Google Scholar]
- 49.Ravens U, Poulet C, Wettwer E, Knaut M. Atrial selectivity of antiarrhythmic drugs. J Physiol. 2013;591:4087–4097. doi: 10.1113/jphysiol.2013.256115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Drici MD, Barhanin J. Cardiac K+ channels and drug-acquired long QT syndrome. Therapie. 2000;55:185–193. [PubMed] [Google Scholar]
- 51.Mitcheson JS, Chen J, Lin M, Culberson C, Sanguinetti MC. A structural basis for drug-induced long QT syndrome. Proc Natl Acad Sci USA. 2000;97:12329–12333. doi: 10.1073/pnas.210244497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fernandez D, Ghanta A, Kauffman GW, Sanguinetti MC. Physicochemical features of the HERG channel drug binding site. J Biol Chem. 2004;279:10120–10127. doi: 10.1074/jbc.M310683200. [DOI] [PubMed] [Google Scholar]
- 53.Ficker E, Jarolimek W, Kiehn J, Baumann A, Brown AM. Molecular determinants of dofetilide block of HERG K+ channels. Circ Res. 1998;82:386–395. doi: 10.1161/01.res.82.3.386. [DOI] [PubMed] [Google Scholar]
- 54.Numaguchi H, Mullins FM, Johnson JP, Jr, et al. Probing the interaction between inactivation gating and Dd-sotalol block of HERG. Circ Res. 2000;87:1012–1018. doi: 10.1161/01.res.87.11.1012. [DOI] [PubMed] [Google Scholar]
- 55.Perrin MJ, Kuchel PW, Campbell TJ, Vandenberg JI. Drug binding to the inactivated state is necessary but not sufficient for high-affinity binding to human ether-a-go-go-related gene channels. Mol Pharmacol. 2008;74:1443–1452. doi: 10.1124/mol.108.049056. [DOI] [PubMed] [Google Scholar]
- 56.Suessbrich H, Schonherr R, Heinemann SH, Lang F, Busch AE. Specific block of cloned Herg channels by clofilium and its tertiary analog LY97241. FEBS Lett. 1997;414:435–438. doi: 10.1016/s0014-5793(97)01030-2. [DOI] [PubMed] [Google Scholar]
- 57.Wu W, Gardner A, Sanguinetti MC. The link between inactivation and high-affinity block of hERG1 channels. Mol Pharmacol. 2015;87:1042–1050. doi: 10.1124/mol.115.098111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gurrola GB, Rosati B, Rocchetti M, et al. A toxin to nervous, cardiac, and endocrine ERG K+ channels isolated from Centruroides noxius scorpion venom. FASEB J. 1999;13:953–962. [PubMed] [Google Scholar]
- 59.Korolkova YV, Kozlov SA, Lipkin AV, et al. An ERG channel inhibitor from the scorpion Buthus eupeus. J Biol Chem. 2001;276:9868–9876. doi: 10.1074/jbc.M005973200. [DOI] [PubMed] [Google Scholar]
- 60.Diochot S, Loret E, Bruhn T, Beress L, Lazdunski M. APETx1, a new toxin from the sea anemone Anthopleura elegantissima, blocks voltage-gated human ether-a-go-go-related gene potassium channels. Mol Pharmacol. 2003;64:59–69. doi: 10.1124/mol.64.1.59. [DOI] [PubMed] [Google Scholar]
- 61.Redaelli E, Cassulini RR, Silva DF, et al. Target promiscuity and heterogeneous effects of tarantula venom peptides affecting Na+ and K+ ion channels. J Biol Chem. 2010;285:4130–4142. doi: 10.1074/jbc.M109.054718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Varro A, Balati B, Iost N, et al. The role of the delayed rectifier component IKs in dog ventricular muscle and Purkinje fibre repolarization. J Physiol. 2000;523:67–81. doi: 10.1111/j.1469-7793.2000.00067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bosch RF, Gaspo R, Busch AE, et al. Effects of the chromanol 293B, a selective blocker of the slow, component of the delayed rectifier K+ current, on repolarization in human and guinea pig ventricular myocytes. Cardiovasc Res. 1998;38:441–450. doi: 10.1016/s0008-6363(98)00021-2. [DOI] [PubMed] [Google Scholar]
- 64.Seebohm G, Chen J, Strutz N, et al. Molecular determinants of KCNQ1 channel block by a benzodiazepine. Mol Pharmacol. 2003;64:70–77. doi: 10.1124/mol.64.1.70. [DOI] [PubMed] [Google Scholar]
- 65.Lengyel C, Iost N, Virag L, et al. Pharmacological block of the slow component of the outward delayed rectifier current (IKs) fails to lengthen rabbit ventricular muscle QTc and action potential duration. Br J Pharmacol. 2001;132:101–110. doi: 10.1038/sj.bjp.0703777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Turgeon J, Daleau P, Bennett PB, et al. Block of IKs, the slow component of the delayed rectifier K+ current, by the diuretic agent indapamide in guinea pig myocytes. Circ Res. 1994;75:879–886. doi: 10.1161/01.res.75.5.879. [DOI] [PubMed] [Google Scholar]
- 67.Heath BM, Terrar DA. Separation of the components of the delayed rectifier potassium current using selective blockers of IKr and IKs in guinea-pig isolated ventricular myocytes. Exp Physiol. 1996;81:587–603. doi: 10.1113/expphysiol.1996.sp003961. [DOI] [PubMed] [Google Scholar]
- 68.Busch AE, Busch GL, Ford E, et al. The role of the IsK protein in the specific pharmacological properties of the IKs channel complex. Br J Pharmacol. 1997;122:187–189. doi: 10.1038/sj.bjp.0701434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sanguinetti MC, Tristani-Firouzi M. hERG potassium channels and cardiac arrhythmia. Nature. 2006;440:463–469. doi: 10.1038/nature04710. [DOI] [PubMed] [Google Scholar]
- 70.Hedley PL, Jorgensen P, Schlamowitz S, et al. The genetic basis of long QT and short QT syndromes: a mutation update. Hum Mutat. 2009;30:1486–1511. doi: 10.1002/humu.21106. [DOI] [PubMed] [Google Scholar]
- 71.Brunner M, Peng X, Liu GX, et al. Mechanisms of cardiac arrhythmias and sudden death in transgenic rabbits with long QT syndrome. J Clin Invest. 2008;118:2246–2259. doi: 10.1172/JCI33578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sanguinetti MC. HERG1 channel agonists and cardiac arrhythmia. Curr Opin Pharmacol. 2014;15:22–27. doi: 10.1016/j.coph.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kang J, Chen XL, Wang H, et al. Discovery of a small molecule activator of the human ether-a-go-go-related gene (HERG) cardiac K+ channel. Mol Pharmacol. 2005;67:827–836. doi: 10.1124/mol.104.006577. [DOI] [PubMed] [Google Scholar]
- 74.Perry M, Sachse FB, Sanguinetti MC. Structural basis of action for a human ether-a-go-go-related gene 1 potassium channel activator. Proc Natl Acad Sci USA. 2007;104:13827–13832. doi: 10.1073/pnas.0703934104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Choi SH, Shin TJ, Hwang SH, et al. Ginsenoside Rg3 decelerates hERG K+ channel deactivation through Ser631 residue interaction. Eur J Pharmacol. 2011;663:59–67. doi: 10.1016/j.ejphar.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 76.Gerlach AC, Stoehr SJ, Castle NA. Pharmacological removal of human ether-a-go-go-related gene potassium channel inactivation by 3-nitro-N-(4-phenoxyphenyl) benzamide (ICA-105574) Mol Pharmacol. 2010;77:58–68. doi: 10.1124/mol.109.059543. [DOI] [PubMed] [Google Scholar]
- 77.Garg V, Stary-Weinzinger A, Sachse F, Sanguinetti MC. Molecular determinants for activation of human ether-a-go-go-related gene 1 potassium channels by 3-nitro-n-(4-phenoxyphenyl) benzamide. Mol Pharmacol. 2011;80:630–637. doi: 10.1124/mol.111.073809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang H, Zou B, Yu H, et al. Modulation of hERG potassium channel gating normalizes action potential duration prolonged by dysfunctional KCNQ1 potassium channel. Proc Natl Acad Sci USA. 2012;109:11866–11871. doi: 10.1073/pnas.1205266109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mannikko R, Bridgland-Taylor MH, Pye H, et al. Pharmacological and electrophysiological characterization of AZSMO-23, an activator of the hERG K+ channel. Br J Pharmacol. 2015;172:3112–3125. doi: 10.1111/bph.13115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Perry M, Sachse FB, Abbruzzese J, Sanguinetti MC. PD-118057 contacts the pore helix of hERG1 channels to attenuate inactivation and enhance K+ conductance. Proc Natl Acad Sci USA. 2009;106:20075–20080. doi: 10.1073/pnas.0906597106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wu W, Sachse FB, Gardner A, Sanguinetti MC. Stoichiometry of altered hERG1 channel gating by small molecule activators. J Gen Physiol. 2014;143:499–512. doi: 10.1085/jgp.201311038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wu W, Gardner A, Sanguinetti MC. Concatenated hERG1 tetramers reveal stoichiometry of altered channel gating by RPR-260243. Mol Pharmacol. 2015;87:401–409. doi: 10.1124/mol.114.096693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zeng H, Lozinskaya IM, Lin Z, et al. Mallotoxin is a novel human ether-a-go-go-related gene (hERG) potassium channel activator. J Pharmacol Exp Ther. 2006;319:957–962. doi: 10.1124/jpet.106.110593. [DOI] [PubMed] [Google Scholar]
- 84.Gessner G, Macianskiene R, Starkus JG, Schonherr R, Heinemann SH. The amiodarone derivative KB130015 activates hERG1 potassium channels via a novel mechanism. Eur J Pharmacol. 2010;632:52–59. doi: 10.1016/j.ejphar.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hansen RS, Diness TG, Christ T, et al. Activation of human ether-a-go-go-related gene potassium channels by the diphenylurea 1,3-bis-(2-hydroxy-5-trifluoromethyl-phenyl)-urea (NS1643) Mol Pharmacol. 2006;69:266–277. doi: 10.1124/mol.105.015859. [DOI] [PubMed] [Google Scholar]
- 86.Casis O, Olesen SP, Sanguinetti MC. Mechanism of action of a novel human ether-a-go-go-related gene channel activator. Mol Pharmacol. 2006;69:658–665. doi: 10.1124/mol.105.019943. [DOI] [PubMed] [Google Scholar]
- 87.Guo J, Cheng YM, Lees-Miller JP, et al. NS1643 interacts around L529 of hERG to alter voltage sensor movement on the path to activation. Biophys J. 2015;108:1400–1413. doi: 10.1016/j.bpj.2014.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Perissinotti LL, Guo J, De Biase PM, et al. Kinetic model for NS1643 drug activation of WT and L529I variants of Kv11.1 (hERG1) potassium channel. Biophys J. 2015;108:1414–1424. doi: 10.1016/j.bpj.2014.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Salata JJ, Jurkiewicz NK, Wang J, et al. A novel benzodiazepine that activates cardiac slow delayed rectifier K+ currents. Mol Pharmacol. 1998;54:220–230. doi: 10.1124/mol.54.1.220. [DOI] [PubMed] [Google Scholar]
- 90.Seebohm G, Pusch M, Chen J, Sanguinetti MC. Pharmacological activation of normal and arrhythmia-associated mutant KCNQ1 potassium channels. Circ Res. 2003;93:941–947. doi: 10.1161/01.RES.0000102866.67863.2B. [DOI] [PubMed] [Google Scholar]
- 91.Gao Z, Xiong Q, Sun H, Li M. Desensitization of chemical activation by auxiliary subunits: convergence of molecular determinants critical for augmenting KCNQ1 potassium channels. J Biol Chem. 2008;283:22649–22658. doi: 10.1074/jbc.M802426200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xiong Q, Sun H, Li M. Zinc pyrithione-mediated activation of voltage-gated KCNQ potassium channels rescues epileptogenic mutants. Nat Chem Biol. 2007;3:287–296. doi: 10.1038/nchembio874. [DOI] [PubMed] [Google Scholar]
- 93.Mattmann ME, Yu H, Lin Z, et al. Identification of (R)-N-(4-(4-methoxyphenyl)thiazol-2-yl)-1-tosylpiperidine-2-carboxamide, ML277, as a novel, potent and selective KV7.1 (KCNQ1) potassium channel activator. Bioorg Med Chem Lett. 2012;22:5936–5941. doi: 10.1016/j.bmcl.2012.07.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Xu Y, Wang Y, Zhang M, et al. Probing binding sites and mechanisms of action of an IKs activator by computations and experiments. Biophys J. 2015;108:62–75. doi: 10.1016/j.bpj.2014.10.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yu H, Lin Z, Mattmann ME, et al. Dynamic subunit stoichiometry confers a progressive continuum of pharmacological sensitivity by KCNQ potassium channels. Proc Natl Acad Sci USA. 2013;110:8732–8737. doi: 10.1073/pnas.1300684110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Abitbol I, Peretz A, Lerche C, Busch AE, Attali B. Stilbenes and fenamates rescue the loss of IKs channel function induced by an LQT5 mutation and other IsK mutants. EMBO J. 1999;18:4137–4148. doi: 10.1093/emboj/18.15.4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zheng Y, Zhu X, Zhou P, et al. Hexachlorophene is a potent KCNQ1/KCNE1 potassium channel activator which rescues LQTs mutants. PLoS One. 2012;7:e51820. doi: 10.1371/journal.pone.0051820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mruk K, Kobertz WR. Discovery of a novel activator of KCNQ1-KCNE1 K channel complexes. PLoS One. 2009;4:e4236. doi: 10.1371/journal.pone.0004236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liin SI, Silvera Ejneby M, Barro-Soria R, et al. Polyunsaturated fatty acid analogs act antiarrhythmically on the cardiac IKs channel. Proc Natl Acad Sci USA. 2015;112:5714–5719. doi: 10.1073/pnas.1503488112. [DOI] [PMC free article] [PubMed] [Google Scholar]