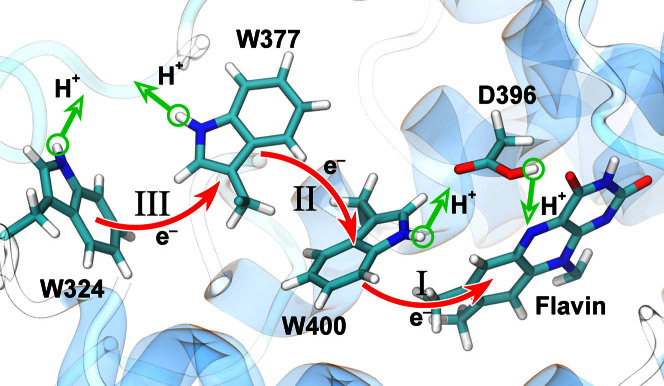
Figure 1. Electron and proton transfers in cryptochrome.
Shown is the flavin cofactor, the tryptophan triad (W400, W377, W324) and the D396 residue forming the active site of cryptochrome-1 from Arabidopsis thaliana. Electron (red) and proton (green) transfer processes that likely govern cryptochrome activation are presented by arrows. The three electron transfers W400 → flavin, W377 → W400·+, and W324 → W377·+ are labeled I, II, and III, respectively. Electron transfer following flavin photo-excitation leads to a radical pair state of cryptochrome: transfer I to [FAD·− + W400·+] with the averaged pair separation of 7.4 Å, sequential transfer I + II to [FAD·− + W377·+] with pair separation of 12.2 Å, sequential transfer I + II + III to [FAD·− + W324·+] with pair separation of 18.2 Å.