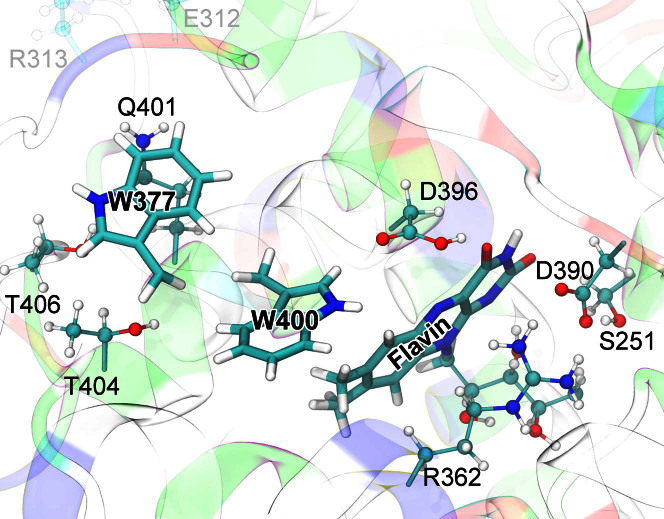
Figure 2. Cryptochrome active site model.
The quantum chemical description of the W377 → W400·+ electron transfer in cryptochrome (electron transfer II in Fig. 1) includes electronic degrees of freedom of riboflavin and of the side chains of amino acid residues S251, R362, W377, D390, D396, Q401, W400, T404, and T406. Residues donating and accepting the electrons (flavin, W400, W377) are shown in licorice representation, while the atoms of coordinating side chains are shown as balls and sticks. The coloring of cryptochrome secondary structure illustrates the charge distribution of the protein's amino acids: positive (blue), negative (red), polar uncharged (green), and hydrophobic uncharged (white). Sidechain groups of all polar, positively and negatively charged amino acids surrounding flavin, W400, and W377 were included into the active site model to describe environmental effects on the W377 → W400·+ electron transfer; distant charged sidechains, e.g., E312, R313 (transparent), were not included in the quantum chemical description.