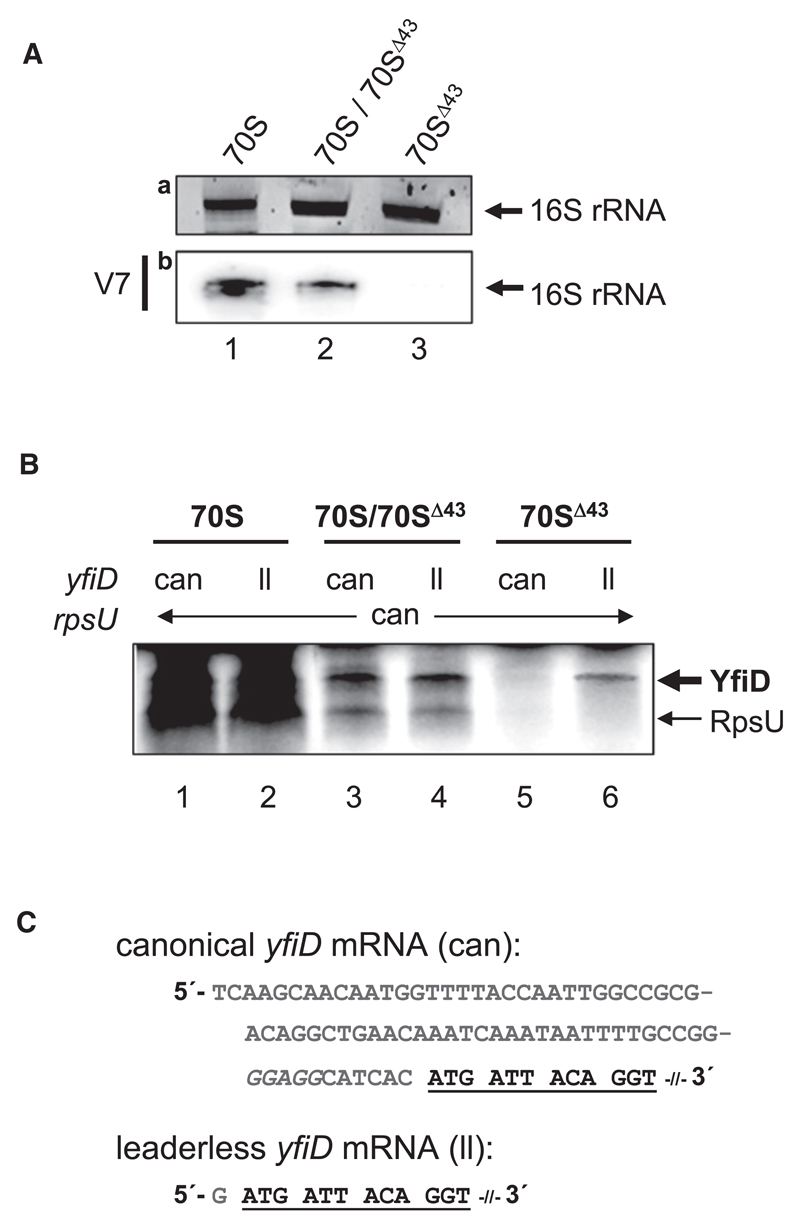
Figure 4. Stress Ribosomes Formed upon Overexpression of mazF In Vivo Selectively Translate Leaderless yfiD mRNA.
(A) rRNA prepared from 10 pmoles of ribosomes purified from untreated cells (70S; lane 1) upon overexpression of mazF (70S/70SΔ43; lane 2) and upon further removal of uncleaved ribosomes employing a biotinylated SD-oligonucleotide (70SΔ43; lane 3), which were used for in vitro translation shown in (B), was separated by denaturing PAGE and stained with ethidium bromide (a) to determine amount and integrity of 16S rRNA. The same rRNA was probed using labeled oligonucleotide V7 (b) to verify presence and absence of the 3′-terminal 43 nt fragment in the individual ribosome preparations.
(B) In vitro translation of canonical (can; lanes 1, 3, and 5) and leaderless (ll; lanes 2, 4, and 6) variants of yfiD mRNA employing 70S ribosomes purified from untreated E. coli MC4100relA+ cells harboring plasmid pSA1 (70S; lanes 1 and 2), purified upon mazF overexpression (70S/70SΔ43; lanes 3 and 4) and upon removal of uncleaved ribosomes employing immobilized biotinylated SD oligonucleotides (70SΔ43; lanes 5 and 6). In all reactions, equimolar amounts of canonical rpsU mRNA were added as internal control. Positions of YfiD (14.3 kD) and RpsU (8.5 kD) proteins in the autoradiograph are indicated to the right.
(C) 5′UTR and proximal coding region (underlined) of canonical and leaderless yfiD mRNAs used for in vitro translation. The SD sequence of the canonical mRNA is indicated in italics.
See also Figure S3.