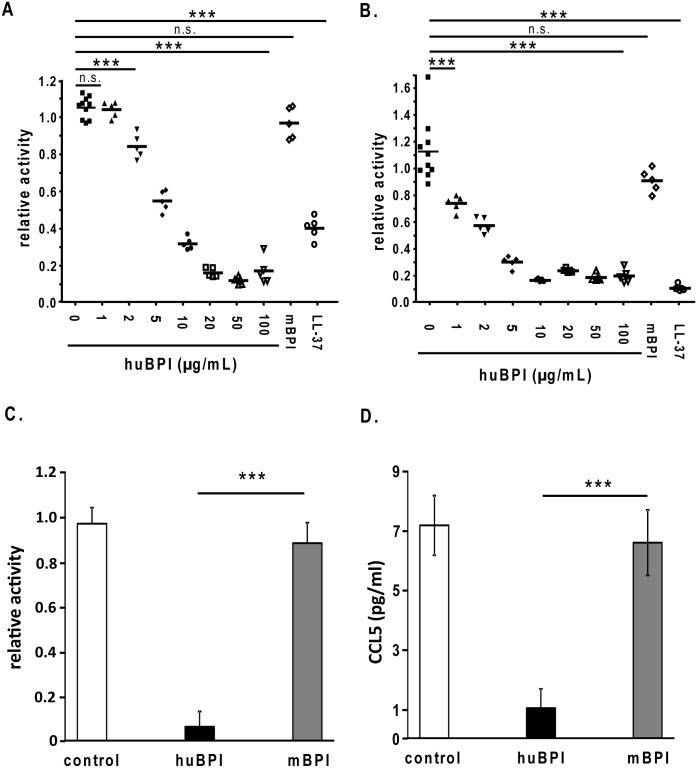
Fig 3. Human BPI-peptide specifically inhibits the replication of different IAV strains.
Protease-deficient MDCK(H) cells were infected with 500 PFU/well of Influenza A virus strain A/PR/8/34 (H1N1) (A) and strain A/Aichi/2/68 (H3N2) (B) for 1 h. After the infection the virus containing supernatant was removed and the cells were grown for additional 13 h. Thereafter, the fixed and permeabilized cells were incubated with the mouse anti–nucleoprotein IAV monoclonal antibody. The binding of the antibody was detected by a donkey anti-mouse IgG-HRP antiserum and adding the reagent TMB Super Sensitive One Component HRP Microwell Substrate. Substrate conversion was detected by 450 nm. For the control sample is n = 8 ± SEM, all other samples n = 5 ± SEM. C) Calu-3 cells were infected with 300 PFU/well of Influenza A virus strain A/Aichi/2/68 (H3N2). Prior to the infection the virus was incubated in the presence or absences of the indicated amount of human or mouse BPI-peptide for 1 h. Thereafter, the virus solution was removed and the cells were incubated for 24 h. The virus amount in the supernatant was analysed by adding an aliquot of the supernatant to MDCK (H) cells for 1 h. After the infection the virus containing supernatant was removed and the cells were grown for additional 13 h. Thereafter, the fixed and permeabilized cells were incubated with the mouse anti–nucleoprotein IAV monoclonal antibody and detected as outlined above. (D) Furthermore, also the release of CCL5 into the supernatant of the infected Calu-3 cells was determined via a specific ELISA. One representative experiment out of 5 performed is displayed. Statistically significant differences are given as p values (** <0.01 and *** <0.001); n.s. is not significant; n = 3 ± SEM.