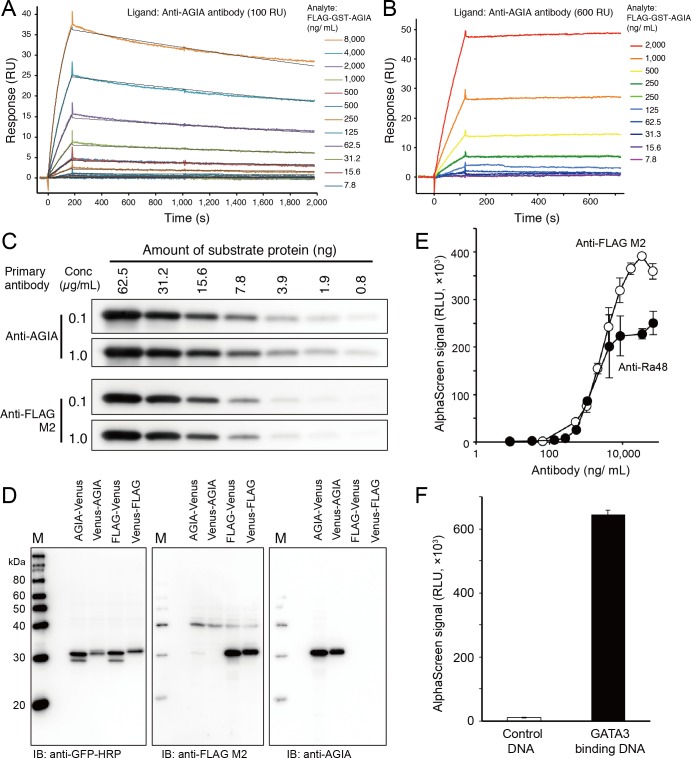
Fig 3. Biochemical characterization of AGIA tag.
(A) Kinetics assay of AGIA tag and anti-AGIA antibody. Anti-AGIA antibody was captured on a protein G-immobilized Biacore sensorchip at 100 RU. Purified FLAG-GST-AGIA protein was then injected for 180 sec as analyte. Black lines represent a global fit of a 1:1 interaction model to each kinetic data set. (B) Stable capturing of AGIA-tagged protein by anti-AGIA antibody. Anti-AGIA antibody was captured on a protein G-immobilized Biacore sensorchip at 600 RU. Purified FLAG-GST-AGIA protein was then injected as analyte for 180 sec at 30 μL/min. (C) High sensitivity of AGIA tag in immunoblotting. FLAG-GST-AGIA protein was synthesized by cell-free system and purified using glutathione sepharose. Concentration of the purified protein was determined by extinction coefficient method [46]. Decreasing amounts of purified FLAG-GST-AGIA were applied to the immunoblot and detected by the antibodies at the concentrations shown. (D) Specific detection of recombinant AGIA-tagged protein by anti-AGIA antibody. AGIA (AGIA-Venus or Venus-AGIA) or FLAG tag (FLAG-Venus or Venus-FLAG) fused Venus proteins were synthesized by cell-free system and applied to immunoblotting. (E) Performance of AGIA tag in protein-protein interaction assay. FLAG- or AGIA-tagged p53 was mixed with biotin-Mdm2, and interaction between the proteins was detected with AlphaScreen using various concentrations of anti-FLAG M2 or anti-AGIA antibodies. (F) Performance of AGIA tag in protein-DNA interaction assay. AGIA-tagged GATA3 protein was mixed with biotinylated oligonucleotides containing GATA3 binding sequence, and the binding was detected with AlphaScreen.