Figure 3.
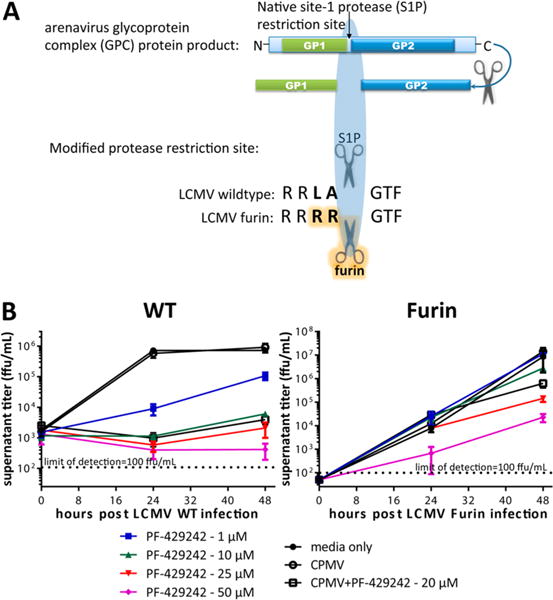
CPMV-PF429242 protection against viral infection. (A) Genetic complementation approach to determine specificity of PF-429242 for S1P cleavage of arenavirus glycoprotein. A mutant LCMVFurin virus was reverse engineered to bear the ubiquitously expressed serine protease furin recognition site, RRRR, rather than the S1P recognition site, RRLA, to enable processing of the viral glycoprotein by furin. (B) BHK-21 cells were infected with either LCMVWT (left) or LCMVFurin (right), and the efficacy of PF-429242, free or loaded in CPMV, was tested. The indicated amounts of the S1P inhibitor PF-429242 or CPMV (empty or loaded with PF-429242 to produce 20 μM final concentration of the S1P inhibitor) were then added to the cultures after infection. The supernatant was collected and titered at the indicated time points. PF-429242 packaged within CPMV inhibits S1P cleavage-dependent viral replication (left), whereas LCMVFurin can complete its lifecycle even if S1P activity is inhibited by PF-429242 (right). Average of 2 similar experiments is shown.
