Abstract
Purpose of Review
This review is written from the perspective of the pediatric clinician involved in the care of premature infants at risk for pulmonary hypertension (PH). The main objective is to better inform the clinician in the diagnosis and treatment of PH in premature infants by reviewing the available relevant literature and focusing on the areas for which there is the greatest need for continued research.
Recent Findings
Continued knowledge regarding the epidemiology of PH in the premature infant population has aided better diagnostic screening algorithms. Included in this knowledge, is the association of PH in infants with bronchopulmonary dysplasia (BPD). However, it is also known that beyond BPD, low birth weight and other conditions that result in increased systemic inflammation are associated with PH. This information has led to the recent recommendation that all infants with BPD should have an echocardiogram to evaluate for evidence of PH prior to discharge from the NICU.
Summary
PH can be a significant comorbidity for premature infants. This review aims to focus the clinician on the available literature to improve recognition of the condition to allow for more timely interventions.
Keywords: Pulmonary hypertension (PH), Prematurity, Bronchopulmonary dysplasia (BPD)
Introduction
Infants born prematurely are at risk to develop bronchopulmonary dysplasia (BPD). Since its original description in the late 1960’s, the definition of BPD has changed dramatically from a description of stiff surfactant deficient lungs that were damaged by conventional ventilators (1) to a more modern definition that describes lung growth arrest and decreased alveolarization (2, 3). Many medical advances have led to this transition in the description of BPD and concurrent with this change is a greater number of premature infants surviving to hospital discharge, though not without significant morbidity (4).
Pulmonary hypertension (PH) is increasingly recognized as a cause of significant morbidity and even mortality in premature infants. Infants with BPD are at even greater risk for PH (5, 6)*. In addition, recent data suggest that detection of early PH is a predictor for greater severity of BPD (7)*. A correlation between the diagnosis of BPD and PH is not surprising, given that alveolarization during lung growth requires pulmonary vascular development (8). Consequently, understanding the pathophysiology of PH in the BPD population involves an understanding of the interactions of these two processes as well as how other factors such as inflammation can influence these interactions (9).
Recent guidelines now recommend that all infants with BPD should be screened with an echocardiogram for PH (10)**. However, there is still much to be learned in terms of the detection and management of infants with BPD-associated PH (BPD-PH). Current practice focuses on minimizing secondary insults to the developing lungs as well as maximizing nutrition to support optimal growth of the infant (8). PH-specific medications are used in this infant population, but there is little evidence to guide best practice in medication selection and dosing (11).
In this review, we discuss the current understanding of PH in the BPD population. By highlighting the current understanding of pathophysiology, epidemiology, diagnostics, and treatment we hope to also highlight the areas where more research is desperately needed to improve the health of this very vulnerable population.
Pathophysiology of PH in BPD
Understanding of the pathophysiology of BPD-PH begins with an understanding of how the pulmonary vasculature develops and functions in utero. While there are still many questions to answer, it is widely accepted that the pulmonary blood vessels develop alongside the airways and throughout development the lung endoderm and vasculature mesoderm are interacting through molecular pathways (9, 12, 13). Vascular endothelial growth factor (VEGF) is very important for signaling of vascular growth and endothelial cell proliferation (14, 15) and this endothelial cell signaling closely interacts with signaling for lung development. Fibroblast growth factors are important regulators of lung development (16) and some of these growth factors are involved with VEGF signaling in capillary development in the distal lung (17). In summary, pulmonary growth and vascular development occurs together through a complex interaction of multiple signaling cascades.
During the in utero process of distal lung growth and vascular development, the pulmonary vascular system is in a state of high vascular resistance. This physiological state facilitates the diverting of the majority of pulmonary blood flow away from the lungs to other organs of the developing fetus. However, after birth, with establishment of an air-liquid-interface, ventilation and an increase in oxygen tension, pulmonary blood flow increases 8-10 fold and pulmonary arterial pressure decreases by half within the few days of life (18). However, in the context of prematurity, alveolarization is incomplete and pulmonary microvascular development is arrested (11, 19). Growth arrest results in decreased cross-sectional area for blood flow and increased pulmonary vascular resistance (PVR). In the context of increased PVR, vasoreactivity is also altered, vascular remodeling occurs, and metabolic function is impaired (8, 20). Prematurity with arrest of pulmonary vascular growth can be further complicated by prenatal insults to the developing pulmonary vasculature.
Causes of prenatal injury include intrauterine growth restriction, infection with resulting inflammation, and conditions that decrease placental blood flow, such as preeclampsia (10). A retrospective cohort study of 138 infants less than 28 weeks gestational age, demonstrated that a low birth weight-for-gestational age was an important predictor of BPD-PH in infants with moderate-to-severe BPD (21). A retrospective cohort study of 98 preterm infants with BPD found that oligohydramnios was a specific risk factor for BPD-PH in preterm infants with moderate or severe BPD (22). Thus, oligohydramnios may be a risk factor for PH owing to alterations in alveolarization, lung development, and as a consequence compromised pulmonary vascular development. Alterations in placental blood flow, such as in preeclampsia, can also affect fetal growth, which can increase the risk for PH in premature infants as already discussed. In addition, alterations in placental blood flow can alter developmental vascular signaling as has been suggested in some animal models (23, 24). These studies serve to highlight some of the possible risk factors for the development of PH in the premature infant population and may represent considerations for PH risk stratification and potential therapeutic targets.
Postnatal factors implicated as causes for increasing a premature infant’s risk of developing PH include poor growth, infection, mechanical ventilation, and altered hemodynamics as with a patent ductus arteriosus (PDA) (8). Inflammation, whether from infection or other exposures, has been implicated in both animal models and human observational studies as a risk factor for the development of PH. More specifically, animal models have shown that reactive oxygen species (ROS) alter hemodynamics by decreasing normal pulmonary artery relaxation to vasodilators and causing vascular remodeling (25, 26). In addition, mechanical ventilation and oxygen therapy can also affect angiogenic signaling and gene expression(20) and increase ROS generation. Altered hemodynamics, including systemic to pulmonary vasculature shunting, can result in pulmonary vascular stress that can lead to vascular remodeling and increased risk of pulmonary hypertension (27, 28).
In summary, lung growth and pulmonary vascular development occur concomitantly through interacting signaling cascades such that alterations in one process can often alter the other. Multiple factors, both before and after birth, can affect lung and pulmonary vascular development as represented in Figure 1. These observations provide opportunities for risk-stratification as well as points for therapeutic intervention.
Figure 1.
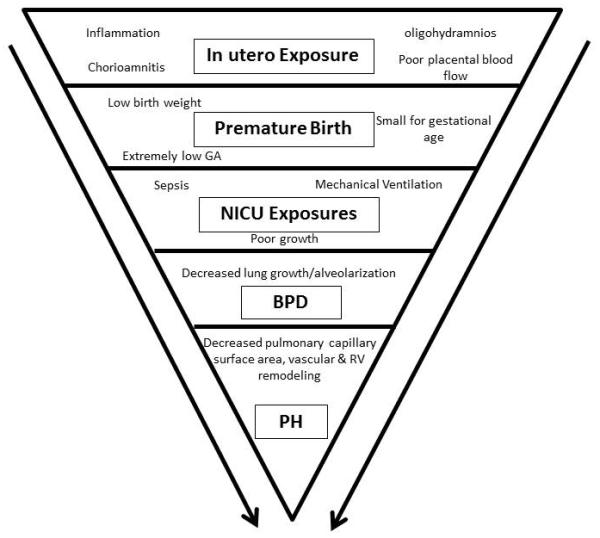
Risk factors for pulmonary hypertension in the premature infant population, and potential opportunities for treatment intervention and areas for further research.
Epidemiology and prognosis of PH in the Premature Infant Population
Due to advances in neonatal care, extremely premature infants (gestational age 22-28 weeks) are surviving to hospital discharge more than in the past (4). While survival is accompanied by multiple challenges, most of these infants do not develop PH. However, for those who develop PH the morbidity and mortality is high. Consequently, efforts are underway to better elucidate the epidemiology of PH in premature infants so as to identify preventable risk factors that predispose the few to long-term significant PH.
PH complicates BPD in 10-30% of cases and BPD accounts for about 5% of childhood PH (5, 6, 22, 29). For most infants, symptoms of PH resolve within the first year of life as respiratory symptoms concurrently improve. However, this is not the case for all infants and there is evidence that the presence of PH does increase the risk of mortality (30). A recent review of a Danish cohort found that 82% of premature infants with PH had moderate/severe BPD,(6) and a similar incidence was recently reported in a cohort in the US (31). Thus, there is recognition that BPD is a risk factor for development of PH in the premature infant population; and PH risk appears to increase with increasing BPD severity.
However, determining the true incidence of PH in the context of BPD is confounded by variability in the definition of BPD (32). In addition, some premature infants develop PH even in the absence of BPD. Regardless of BPD status, increasing days on mechanical ventilation and a greater length of stay have been associated with PH in the premature infant population (6). Therefore, in addition to the recent guidelines (10) suggesting evaluation for PH in the BPD population, it may be reasonable to consider screening a broader cohort of premature infants with other risk factors.
Evaluation of PH in the Premature Infant Population
Cardiac catheterization is considered the gold standard for the diagnosis of PH, which is typically defined as a mean pulmonary artery pressure over 25 mmHg in children and adults regardless of age. Furthermore, pediatric pulmonary hypertensive vascular disease has been used as a term to identify children with PH accompanied by elevated PVR (pulmonary vascular resistance index >3.0 Wood units m2 for biventricular circulations), which is the type of PH typically seen in premature infants (33). But in the vulnerable premature infant population with little pulmonary reserve the risks of cardiac catheterization often force clinicians to rely on non-invasive metrics of PH, such as the transthoracic echocardiogram. Echocardiograms provide a tremendous amount of diagnostic information regarding PH, but there is a significant need for standardization of parameters in the BPD population (34).
The standard evaluation of PH by echocardiogram focuses on parameters related to the right ventricle and when present the tricuspid regurgitant jet velocity (TRJV) is a useful parameter to estimate the right ventricular systolic pressure (RVSP) (35). However, in many cases an accurate estimate of RVSP is not always possible as tricuspid regurgitation may be undetectable or TRJV may be incomplete and thus alternative parameters are employed (34). Consequently, there is a need for other echocardiographic parameters. Nagiub and colleagues recently reviewed the literature and proposed an echocardiogram screening protocol for infants with BPD (34)*. Overall, the proposed protocol suggests using parameters such as PDA blood flow, left pulmonary artery flow analysis, and septal geometry when TRJV cannot be accurately estimated.
In addition, there is also recent data that suggests that echocardiogram evidence of PH at 7 days of life predicts the development of BPD with late PH in preterm infants (7). One of the implications of this report is that predicting which infants are at risk for the later development of BPD-PH very early in life (36) may be possible. This report underscores the need for screening for PH both early and late in the NICU course, as lung growth and vascular development are interdependent from the beginning of development.
There is thus a wealth of information accessible by echocardiogram. In pediatric populations, especially the BPD population, the biggest issue is lack of standardization of these parameters. Because cardiac catheterization is not routinely performed in the BPD population many of these echocardiogram parameters are necessary proxies for PH. However, the fidelity between cardiac catheterization and echocardiogram findings is often less than optimal (37). Furthermore, there are technical challenges to performing echocardiograms in these small premature infants and there is often considerable inter-observer variability. Overall, there is need for greater standardization of echocardiogram parameters in the BPD population.
Other testing can be used to help risk stratify infants with concerns for PH, including serum B-type natriuretic peptide (BNP) levels, which is elevated in individuals with conditions of cardiac strain. BNP levels are elevated in children and adults with PH. In the context of premature infants with BPD-PH, elevated BNP level is associated with an increased risk of mortality (38). However, an elevated BNP level is relatively nonspecific and may have the greatest utility as a laboratory parameter to follow longitudinally. Cardiac MRI represents another promising non-invasive modality for the diagnosis of PH (39), but this testing is also expensive, time consuming, and in the BPD population frequently require sedation, which is not without risk. Again, in this very vulnerable population it seems most appropriate to continue to focus efforts on improving and standardizing non-invasive assessments of pulmonary hypertension.
Treatment and Management of Pulmonary Hypertension in the BPD Population
Rather than pharmacologic interventions, the first step in the management and treatment of PH in the BPD population is attention to interventions to protect the developing lungs from future insults and supporting optimal lung growth. The pathophysiology of PH in infants with BPD relates to decreased lung growth, specifically alveolarization, and arrested pulmonary vascular development which ultimately leads to decreased pulmonary capillary surface area and consequently pulmonary hypertension. However, we know that alveolarization is an active process that continues beyond the first year of life (3). When alveolarization improves so does pulmonary capillary development and consequently promoting optimal lung growth is a key component of PH management in the BPD population. A significant part of this includes promoting optimal nutrition and monitoring for adequate weight gain. As also already discussed, infants that are small for gestational age are at increased risk for the development of PH and thus optimal lung growth is of extreme importance in these very vulnerable infants.
In addition to promoting growth, protecting the developing lungs from secondary insults that can worsen PH is also important. Clinical situations such as gastro-esophageal reflux and dysphagia with aspiration can worsen lower airway inflammation, promote ventilation-perfusion (V/Q) mismatch, and consequently worsen PH. In addition, infection prevention interventions such as influenza immunization, is also important as infants with BPD and PH can experience respiratory failure in the setting of acute viral infections.
PH-specific medications have emerged for the care of PAH in adults over the past 20 years, and applied to children. These include three broad classes of medications: (1) prostacylins and prostacyclin derivatives; (2) modulators of the nitric oxide pathway; (3) inhibitors of endothelin-related vasoconstriction (40). PH-specific medications are used frequently in the BPD population, but overall there is a lack of consensus as to the best practice with these medications.
In the acute setting of a hospitalized neonate, inhaled nitric oxide is often first-line therapy to improve oxygenation through pulmonary vasodilation, and is the only FDA-approved agent for the management of any form of PH in children (although not specifically approved in the BPD population) (41). This medication has the advantage of a rapid onset of significant pulmonary vasodilation without systemic vasodilation, but it is not generally applied over the long-term PH management. Of the available medications, sildenafil has the most evidence for its use in the BPD population, but most of this evidence is retrospective case reviews (42). Sildenafil is a phosphodiesterase-5 inhibitor that prevents breakdown of cyclic GMP and thus augments the efficacy of endogenous nitric oxide to promote pulmonary vasodilatation. There is evidence that sildenafil improves echocardiographic and clinical parameters of BPD-PH, but there are questions persist regarding the long-term clinical benefit of the medication. A recent recommendation by the FDA was countered by a published reply written by several pediatric PH clinicians in North America (43-45). Recent dosing recommendations for this medication often focuses on reaching lower dose target levels (2-3 mg/kg/day frequently divided as a dose given 3-4 times per day), rather than higher doses (up to 8 mg/kg/day) as was originally reported in the literature due to safety concerns (42, 46, 47).
Interestingly, recent multi-center data report that the median duration of sildenafil therapy in the BPD population is about 50 days, suggesting that long-term exposure is a rare need (48). This short duration of therapy is in agreement with the natural history of most infants with BPD-PH in which PH tends to resolve within the first year of life. However, this should not be taken as proof of clinical efficacy. Currently, there is no clear evidence that sildenafil directly enhances lung growth and/or vascular growth. Clearly, there is urgency surrounding the need for continued research in the field.
For some infants with BPD and PH, the severity of their pulmonary vascular disease warrants therapy beyond monotherapy with sildenafil. While endothelin antagonists, such as bosentan, or prostacyclin analogues are sometimes used, efficacy and dosing data in the BPD population is limited (10)*. In our experience, escalation of PH-specific therapy beyond monotherapy should trigger a focused evaluation of comorbid conditions and concurrent therapeutic exposures, and strong consideration for cardiac catheterization prior to addition of another PH-specific medication.
Conclusions
In summary, PH often complicates BPD wherein lung growth and alveolarization is compromised by impaired vascular development that decreases pulmonary capillary cross-sectional area and increases pulmonary vascular resistance. Infants with BPD are at increased risk for the development of PH and echocardiographic evaluation is essential even as more standardized approaches to interpretation of echocardiophic data are pursued. Treatment entails promoting lung growth, ensuring adequate oxygenation and ventilation and minimizing secondary insults to the lungs. When PH-specific therapy is initiated the first-line choice is sildenafil; however, there is a significant need for research to guide dosing and use of this medication. Other PH-specific medications are sometimes employed in this population, but there is even less evidence to guide these medications. While for most infants with BPD and PH, the natural history is resolution of PH by the first year of life, the prognosis for children with ongoing PH is significantly less favorable (5, 49). Future research needs to continue to focus on early identification of these infants as well as best treatment practices.
Key Points.
The pathophysiology of PH in the premature infant involves decreased lung growth and alveolarization with concurrent decreased pulmonary vascular growth that results in decreased pulmonary capillary surface area.
Bronchopulmonary dysplasia (BPD) is associated with the development of pulmonary hypertension (PH) in the premature infant population.
In addition to BPD, low birth weight and causes of increased inflammation have been associated with the development of PH.
It is now recommended that all infants with BPD have an echocardiogram to evaluate for evidence of PH prior to discharge from the NICU.
There is need for greater evidence regarding best treatment practices, but routine care starts with minimizing secondary insults to the developing lungs and promotion of optimal growth.
Acknowledgements
None
Financial support and sponsorship:
This work was supported by NIH T32 GM 07569 in clinical pharmacology (O’Connor), and NIH K23 HL 098743 (Austin).
Footnotes
Conflict of interest:
None
BIBLIOGRAPHY & REFERENCES CITED
- 1.Northway WH, Jr., Rosan RC, Porter DY. Pulmonary disease following respirator therapy of hyaline-membrane disease. Bronchopulmonary dysplasia. The New England journal of medicine. 1967 Feb 16;276(7):357–68. doi: 10.1056/NEJM196702162760701. PubMed PMID: 5334613. [DOI] [PubMed] [Google Scholar]
- 2.Kinsella JP, Greenough A, Abman SH. Bronchopulmonary dysplasia. The Lancet. 2006;367(9520):1421–31. doi: 10.1016/S0140-6736(06)68615-7. [DOI] [PubMed] [Google Scholar]
- 3.Silva DMN,C, Pozarska A, Morty RE. Recent Advances in the Mechanisms of Lung Alveolarization and the Pathogenesis of Bronchopulmonary Dysplasia. American journal of physiology Lung cellular and molecular physiology. 2015;309:L1239–L72. doi: 10.1152/ajplung.00268.2015. [DOI] [PubMed] [Google Scholar]
- 4.Stoll BJ, Hansen NI, Bell EF, Walsh MC, Carlo WA, Shankaran S, et al. Trends in Care Practices, Morbidity, and Mortality of Extremely Preterm Neonates, 1993-2012. Jama. 2015 Sep 8;314(10):1039–51. doi: 10.1001/jama.2015.10244. PubMed PMID: 26348753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khemani E, McElhinney DB, Rhein L, Andrade O, Lacro RV, Thomas KC, et al. Pulmonary artery hypertension in formerly premature infants with bronchopulmonary dysplasia: clinical features and outcomes in the surfactant era. Pediatrics. 2007 Dec;120(6):1260–9. doi: 10.1542/peds.2007-0971. PubMed PMID: 18055675. Epub 2007/12/07. eng. This retrospective case-series of 42 premature infants from 1998-2006 is one of the initial manuscripts to describe PH in the setting of a more modern BPD population. This manuscript includes hemodynamic parameters and survival data of the premature infants who developed pulmonary hypertension.
- 6.Ali ZS, Peter, Dodd James, Jeppesen Dorthe Lisbeth. Predictors of bronchopulmonary dysplasia and pulmoanry hypertension in newborn children. Danish Medical Journal. 2013;60(8):1–5. This large retrospective study of 400 premature infants over a 10 year period describes the incidence and clinical characteristics of the premature infants who developed PH. It also highlights the association of BPD with PH as well as the association of PH with other respiratory morbidities, such as longer duration of positive pressure with PH. It is important because it helps to better characterize the premature infant at risk for the development of PH.
- 7.Mourani PM, Sontag MK, Younoszai A, Miller JI, Kinsella JP, Baker CD, et al. Early pulmonary vascular disease in preterm infants at risk for bronchopulmonary dysplasia. American journal of respiratory and critical care medicine. 2015 Jan 1;191(1):87–95. doi: 10.1164/rccm.201409-1594OC. PubMed PMID: 25389562. Pubmed Central PMCID: 4299632. This prospective longitudinal study identified the relationship between early in life echocardiograms and subsequent development of both BPD and PH.
- 8.Mourani PM, Abman SH. Pulmonary Hypertension and Vascular Abnormalities in Bronchopulmonary Dysplasia. Clinics in perinatology. 2015 Dec;42(4):839–55. doi: 10.1016/j.clp.2015.08.010. PubMed PMID: 26593082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kool HM,D, Tibboel D, Klein A, Rottier R. Pulmonary Vascular Development Goes Awry in Congenital Lung Abnormalities. Birth Defects Res C Embryo Today. 2014;102(4):343–58. doi: 10.1002/bdrc.21085. [DOI] [PubMed] [Google Scholar]
- 10.Abman SH, Hansmann G, Archer SL, Ivy DD, Adatia I, Chung WK, et al. Pediatric Pulmonary Hypertension: Guidelines From the American Heart Association and American Thoracic Society. Circulation. 2015 Nov 24;132(21):2037–99. doi: 10.1161/CIR.0000000000000329. PubMed PMID: 26534956. These recent guidelines highlight epidemiology, diagnostic, and treatment recommendations for pediatric PH, including the premature infant population. Specifically, these guidelines include the recommendation that all infants with BPD have an echocardiogram to evaluate for PH at 36 weeks corrected gestational age.
- 11.Wardle AJ, Wardle R, Luyt K, Tulloh R. The utility of sildenafil in pulmonary hypertension: a focus on bronchopulmonary dysplasia. Archives of disease in childhood. 2013 Aug;98(8):613–7. doi: 10.1136/archdischild-2012-303333. PubMed PMID: 23625986. [DOI] [PubMed] [Google Scholar]
- 12.Morrisey EE, Hogan BL. Preparing for the first breath: genetic and cellular mechanisms in lung development. Developmental cell. 2010 Jan 19;18(1):8–23. doi: 10.1016/j.devcel.2009.12.010. PubMed PMID: 20152174. Pubmed Central PMCID: 3736813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herriges M, Morrisey EE. Lung development: orchestrating the generation and regeneration of a complex organ. Development. 2014 Feb;141(3):502–13. doi: 10.1242/dev.098186. PubMed PMID: 24449833. Pubmed Central PMCID: 3899811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O'Shea KS, et al. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996 Apr 4;380(6573):439–42. doi: 10.1038/380439a0. PubMed PMID: 8602242. [DOI] [PubMed] [Google Scholar]
- 15.Healy AM, Morgenthau L, Zhu X, Farber HW, Cardoso WV. VEGF is deposited in the subepithelial matrix at the leading edge of branching airways and stimulates neovascularization in the murine embryonic lung. Developmental dynamics : an official publication of the American Association of Anatomists. 2000 Nov;219(3):341–52. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1061>3.0.CO;2-M. PubMed PMID: 11066091. [DOI] [PubMed] [Google Scholar]
- 16.Shannon JM, Hyatt BA. Epithelial-mesenchymal interactions in the developing lung. Annual review of physiology. 2004;66:625–45. doi: 10.1146/annurev.physiol.66.032102.135749. PubMed PMID: 14977416. [DOI] [PubMed] [Google Scholar]
- 17.White AC, Lavine KJ, Ornitz DM. FGF9 and SHH regulate mesenchymal Vegfa expression and development of the pulmonary capillary network. Development. 2007 Oct;134(20):3743–52. doi: 10.1242/dev.004879. PubMed PMID: 17881491. Pubmed Central PMCID: 2099314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hooper SB, Polglase GR, Roehr CC. Cardiopulmonary changes with aeration of the newborn lung. Paediatric respiratory reviews. 2015 Jun;16(3):147–50. doi: 10.1016/j.prrv.2015.03.003. PubMed PMID: 25870083. Pubmed Central PMCID: 4526381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lakshminrusimha S, Keszler M. Persistent Pulmonary Hypertension of the Newborn. NeoReviews. 2015 Dec;16(12):e680–e92. doi: 10.1542/neo.16-12-e680. PubMed PMID: 26783388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baker CD, Abman SH, Mourani PM. Pulmonary Hypertension in Preterm Infants with Bronchopulmonary Dysplasia. Pediatr Allergy Immunol Pulmonol. 2014 Mar 1;27(1):8–16. doi: 10.1089/ped.2013.0323. PubMed PMID: 24669351. Pubmed Central PMCID: 3961769. Epub 2014/03/29. Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Check J, Gotteiner N, Liu X, Su E, Porta N, Steinhorn R, et al. Fetal growth restriction and pulmonary hypertension in premature infants with bronchopulmonary dysplasia. Journal of perinatology : official journal of the California Perinatal Association. 2013 Jul;33(7):553–7. doi: 10.1038/jp.2012.164. PubMed PMID: 23328924. Pubmed Central PMCID: 3633609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim DH, Kim HS, Choi CW, Kim EK, Kim BI, Choi JH. Risk factors for pulmonary artery hypertension in preterm infants with moderate or severe bronchopulmonary dysplasia. Neonatology. 2012;101(1):40–6. doi: 10.1159/000327891. PubMed PMID: 21791938. [DOI] [PubMed] [Google Scholar]
- 23.Tang JR, Karumanchi SA, Seedorf G, Markham N, Abman SH. Excess soluble vascular endothelial growth factor receptor-1 in amniotic fluid impairs lung growth in rats: linking preeclampsia with bronchopulmonary dysplasia. American journal of physiology Lung cellular and molecular physiology. 2012 Jan 1;302(1):L36–46. doi: 10.1152/ajplung.00294.2011. PubMed PMID: 22003089. Pubmed Central PMCID: 3349373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Madurga A, Mizikova I, Ruiz-Camp J, Morty RE. Recent advances in late lung development and the pathogenesis of bronchopulmonary dysplasia. American journal of physiology Lung cellular and molecular physiology. 2013 Dec;305(12):L893–905. doi: 10.1152/ajplung.00267.2013. PubMed PMID: 24213917. [DOI] [PubMed] [Google Scholar]
- 25.Wedgwood S, Steinhorn RH. Role of Reactive Oxygen Species in Neonatal Pulmonary Vascular Disease. Antioxidants & redox signaling. 2014 Feb 19; doi: 10.1089/ars.2013.5785. PubMed PMID: 24350610. Epub 2013/12/20. Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rabinovitch M, Guignabert C, Humbert M, Nicolls MR. Inflammation and immunity in the pathogenesis of pulmonary arterial hypertension. Circulation research. 2014 Jun 20;115(1):165–75. doi: 10.1161/CIRCRESAHA.113.301141. PubMed PMID: 24951765. Epub 2014/06/22. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi EK, Jung YH, Kim HS, Shin SH, Choi CW, Kim EK, et al. The Impact of Atrial Left-to-Right Shunt on Pulmonary Hypertension in Preterm Infants with Moderate or Severe Bronchopulmonary Dysplasia. Pediatrics and neonatology. 2015 Oct;56(5):317–23. doi: 10.1016/j.pedneo.2014.12.006. PubMed PMID: 26328892. [DOI] [PubMed] [Google Scholar]
- 28.An HS, Bae EJ, Kim GB, Kwon BS, Beak JS, Kim EK, et al. Pulmonary hypertension in preterm infants with bronchopulmonary dysplasia. Korean circulation journal. 2010 Mar;40(3):131–6. doi: 10.4070/kcj.2010.40.3.131. PubMed PMID: 20339498. Pubmed Central PMCID: 2844979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berger RM, Beghetti M, Humpl T, Raskob GE, Ivy DD, Jing ZC, et al. Clinical features of paediatric pulmonary hypertension: a registry study. Lancet. 2012 Feb 11;379(9815):537–46. doi: 10.1016/S0140-6736(11)61621-8. PubMed PMID: 22240409. Pubmed Central PMCID: 3426911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Slaughter JL, Pakrashi T, Jones DE, South AP, Shah TA. Echocardiographic detection of pulmonary hypertension in extremely low birth weight infants with bronchopulmonary dysplasia requiring prolonged positive pressure ventilation. Journal of perinatology : official journal of the California Perinatal Association. 2011 Oct;31(10):635–40. doi: 10.1038/jp.2010.213. PubMed PMID: 21311503. [DOI] [PubMed] [Google Scholar]
- 31.Aswani R, Hayman L, Nichols G, Luciano AA, Amankwah EK, Leshko JL, et al. Oxygen requirement as a screening tool for the detection of late pulmonary hypertension in extremely low birth weight infants. Cardiology in the young. 2015 Jun 29;:1–7. doi: 10.1017/S1047951115000608. PubMed PMID: 26119883. [DOI] [PubMed] [Google Scholar]
- 32.Islam JY, Keller RL, Aschner JL, Hartert TV, Moore PE. Understanding the Short- and Long-Term Respiratory Outcomes of Prematurity and Bronchopulmonary Dysplasia. American journal of respiratory and critical care medicine. 2015 Jul 15;192(2):134–56. doi: 10.1164/rccm.201412-2142PP. PubMed PMID: 26038806. Pubmed Central PMCID: 4532824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cerro MJ, Abman S, Diaz G, Freudenthal AH, Freudenthal F, Harikrishnan S, et al. A consensus approach to the classification of pediatric pulmonary hypertensive vascular disease: Report from the PVRI Pediatric Taskforce, Panama 2011. Pulm Circ. 2011;1(2):286–98. doi: 10.4103/2045-8932.83456. PubMed PMID: 21874158. Pubmed Central PMCID: 3161725. Epub 2011/08/30. Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nagiub M, Lee S, Guglani L. Echocardiographic assessment of pulmonary hypertension in infants with bronchopulmonary dysplasia: systematic review of literature and a proposed algorithm for assessment. Echocardiography. 2015 May;32(5):819–33. doi: 10.1111/echo.12738. PubMed PMID: 25231322. This recent manuscript reviews the available literature and proposes an algorithm for standardizing echocardiogram reads of PH in the premature infant population.
- 35.Amsallem M, Sternbach JM, Adigopula S, Kobayashi Y, Vu TA, Zamanian R, et al. Addressing the Controversy of Estimating Pulmonary Arterial Pressure by Echocardiography. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 2015 Dec 11; doi: 10.1016/j.echo.2015.11.001. PubMed PMID: 26691401. [DOI] [PubMed] [Google Scholar]
- 36.Farrow KN, Steinhorn RH. Pulmonary hypertension in premature infants. Sharpening the tools of detection. American journal of respiratory and critical care medicine. 2015 Jan 1;191(1):12–4. doi: 10.1164/rccm.201411-2112ED. PubMed PMID: 25551345. Pubmed Central PMCID: 4299635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mourani PM, Sontag MK, Younoszai A, Ivy DD, Abman SH. Clinical utility of echocardiography for the diagnosis and management of pulmonary vascular disease in young children with chronic lung disease. Pediatrics. 2008 Feb;121(2):317–25. doi: 10.1542/peds.2007-1583. PubMed PMID: 18245423. Pubmed Central PMCID: 3121163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cuna A, Kandasamy J, Sims B. B-type natriuretic peptide and mortality in extremely low birth weight infants with pulmonary hypertension: a retrospective cohort analysis. BMC pediatrics. 2014;14:68. doi: 10.1186/1471-2431-14-68. PubMed PMID: 24612708. Pubmed Central PMCID: PMC3975241. Epub 2014/03/13. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alassas K, Mergo P, Ibrahim el S, Burger C, Safford R, Parikh P, et al. Cardiac MRI as a diagnostic tool in pulmonary hypertension. Future cardiology. 2014 Jan;10(1):117–30. doi: 10.2217/fca.13.97. PubMed PMID: 24344668. [DOI] [PubMed] [Google Scholar]
- 40.McLaughlin VV, Shah SJ, Souza R, Humbert M. Management of pulmonary arterial hypertension. J Am Coll Cardiol. 2015 May 12;65(18):1976–97. doi: 10.1016/j.jacc.2015.03.540. PubMed PMID: 25953750. [DOI] [PubMed] [Google Scholar]
- 41.Kumar P. Use of inhaled nitric oxide in preterm infants. Pediatrics. 2014 Jan;133(1):164–70. doi: 10.1542/peds.2013-3444. PubMed PMID: 24379225. Epub 2014/01/01. eng. [DOI] [PubMed] [Google Scholar]
- 42.Bhatt-Mehta V, Donn SM. Sildenafil for pulmonary hypertension complicating bronchopulmonary dysplasia. Expert review of clinical pharmacology. 2014 Jul;7(4):393–5. doi: 10.1586/17512433.2014.922867. PubMed PMID: 24866752. Epub 2014/05/29. eng. [DOI] [PubMed] [Google Scholar]
- 43.Perez KM, Laughon M. Sildenafil in Term and Premature Infants: A Systematic Review. Clinical therapeutics. 2015 Nov 1;37(11):2598–607. e1. doi: 10.1016/j.clinthera.2015.07.019. PubMed PMID: 26490498. [DOI] [PubMed] [Google Scholar]
- 44.Trottier-Boucher MN, Lapointe A, Malo J, Fournier A, Raboisson MJ, Martin B, et al. Sildenafil for the Treatment of Pulmonary Arterial Hypertension in Infants with Bronchopulmonary Dysplasia. Pediatric cardiology. 2015 Aug;36(6):1255–60. doi: 10.1007/s00246-015-1154-0. PubMed PMID: 25824807. [DOI] [PubMed] [Google Scholar]
- 45.Abman SH, Kinsella JP, Rosenzweig EB, Krishnan U, Kulik T, Mullen M, et al. Implications of the U.S. Food and Drug Administration warning against the use of sildenafil for the treatment of pediatric pulmonary hypertension. Am J Respir Crit Care Med. 2013 Mar 15;187(6):572–5. doi: 10.1164/rccm.201210-1928PP. PubMed PMID: 23220921. [DOI] [PubMed] [Google Scholar]
- 46.Barst RJ, Beghetti M, Pulido T, Layton G, Konourina I, Zhang M, et al. STARTS-2: long-term survival with oral sildenafil monotherapy in treatment-naive pediatric pulmonary arterial hypertension. Circulation. 2014 May 13;129(19):1914–23. doi: 10.1161/CIRCULATIONAHA.113.005698. PubMed PMID: 24637559. [DOI] [PubMed] [Google Scholar]
- 47.Barst RJ, Ivy DD, Gaitan G, Szatmari A, Rudzinski A, Garcia AE, et al. A randomized, double-blind, placebo-controlled, dose-ranging study of oral sildenafil citrate in treatment-naive children with pulmonary arterial hypertension. Circulation. 2012 Jan 17;125(2):324–34. doi: 10.1161/CIRCULATIONAHA.110.016667. PubMed PMID: 22128226. [DOI] [PubMed] [Google Scholar]
- 48.Backes CH, Reagan PB, Smith CV, Jadcherla SR, Slaughter JL. Sildenafil Treatment of Infants With Bronchopulmonary Dysplasia-Associated Pulmonary Hypertension. Hospital pediatrics. 2016 Jan;6(1):27–33. doi: 10.1542/hpeds.2015-0076. PubMed PMID: 26666265. [DOI] [PubMed] [Google Scholar]
- 49.del Cerro MJ, Sabate Rotes A, Carton A, Deiros L, Bret M, Cordeiro M, et al. Pulmonary hypertension in bronchopulmonary dysplasia: clinical findings, cardiovascular anomalies and outcomes. Pediatric pulmonology. 2014 Jan;49(1):49–59. doi: 10.1002/ppul.22797. PubMed PMID: 23788443. [DOI] [PubMed] [Google Scholar]


