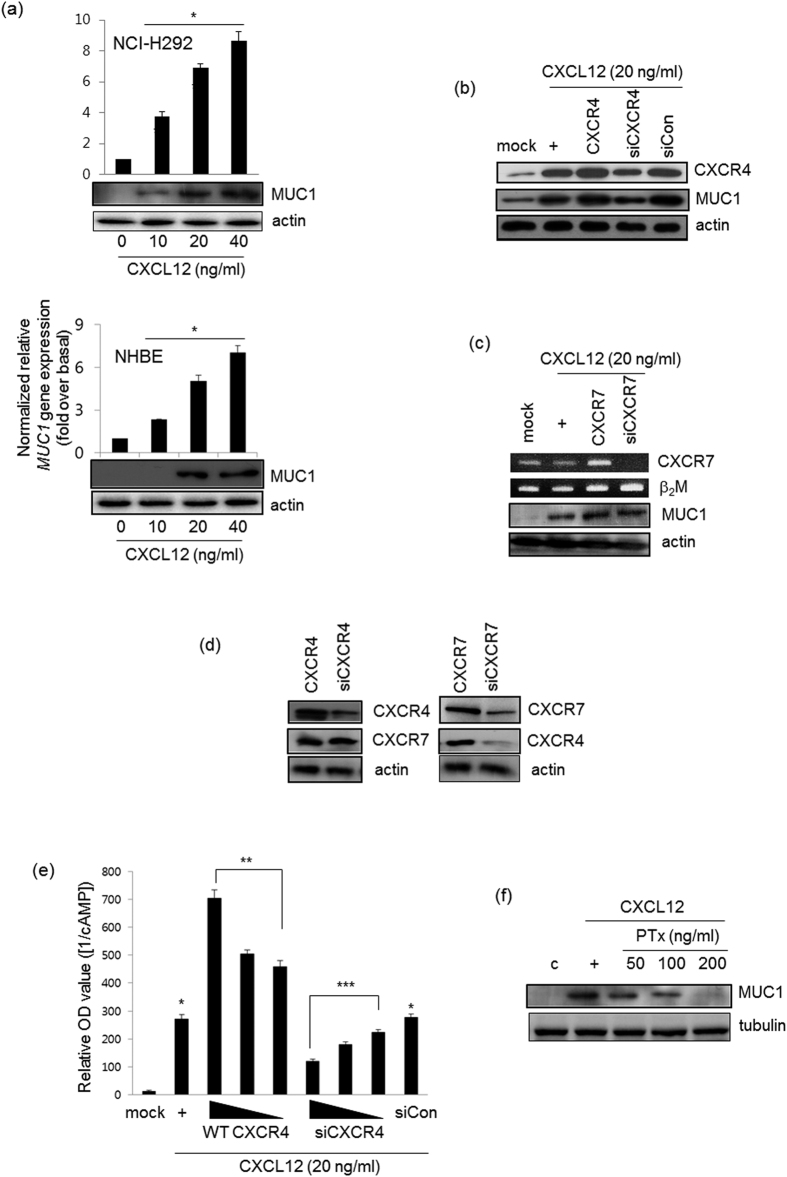
Figure 1. CXCL12 induces MUC1 expression via the CXCR4 receptor in NCI-H292 cells.
(a) Confluent and quiescent NCI-H292 cells (upper panel) and NHBE cells (lower panel) were treated for 24 hours with various concentrations of CXCL12, and then lysates were harvested and analyzed by real-time quantitative RT-PCR and Western blot for MUC1 transcript and protein, respectively. *p < 0.05 compared to the control. After cells were transfected with either construct expressing wild-type CXCR4 or the siRNA constructs of CXCR4 (b,c for CXCR7), cells were treated with CXCL12 for 24 hours prior to the collection of total RNA for conventional RT-PCR (for CXCR4 transcript) and real-time quantitative RT-PCR. *p < 0.05 compared to the control, **p < 0.05 compared to CXCL12 treatment only, and ***p < 0.05 compared to CXCR4 transfection. β2M, Beta-2- microglobulin, was used as a loading control. (d) After transfection with either wild-type CXCR4 or siRNA-CXCR4, cells were harvested and performed Western blot analysis for CXCR7 transcript (left panel). And either wild-type CXCR7 or siRNA-CXCR7 construct was transfected to Western blot for CXCR4 (right panel). (e) The cells were transiently transfected with the construct expressing wild-type CXCR4 (0.1, 0.5, or 1.0 μg) or siRNA-CXCR4 (5, 25, or 50 nM). Cells were serum-starved overnight and then treated with the indicated concentrations of CXCL12 for four hours, after which cAMP production was measured. The values shown are means ± SD of experiments performed in triplicate. *p < 0.05 compared to the control, **p < 0.05 compared to CXCL12 treatment only, and ***p < 0.05 compared to CXCR4 transfection. (f) Cells were treated with PTx with different dosages for 4 hr, and then stimulated for 24 hr with CXCL12 prior to collection of total lysates for Western blot analysis for MUC1 expression with anti-MUC1 against C-terminal region. These figures are representative of three independent experiments. All of uncropped gels (full-length gels) are located in supplemental figures.