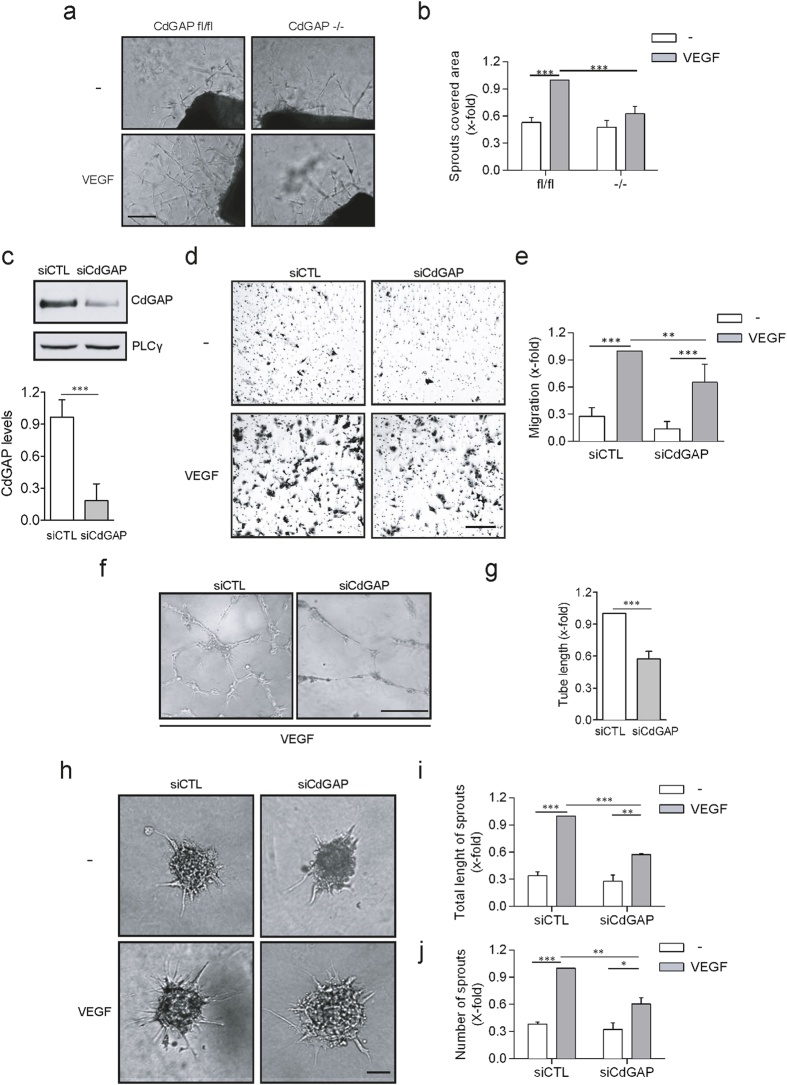
Figure 2. CdGAP is required for VEGF-mediated angiogenesis.
(a) Aorta rings prepared from adult CdGAPfl/fl and CdGAP−/− mice were embedded in Matrigel and cultured in medium containing either VEGF (50 ng/ml) or PBS (−) as a control. Scale bar, 200 μm. (b) The average surface occupied by capillaries sprouting from the aortic rings was quantified using the Metamorph software. n = 5/genotype per condition. Error bars indicate SEM (***P ≤ 0.001, One-way ANOVA, Newman-Keuls post-test). (c) Western blot analysis of CdGAP expression levels in HUVECs electroporated with control siRNAs (siCTL) and CdGAP siRNAs (siCdGAP). PLCγ was used as a protein loading control. CdGAP expression levels were quantified by densitometric analysis. TCL, total cell lysate. n = 4, error bars indicate SEM (***P ≤ 0.001, Unpaired student’s t-test). (d) Control and CdGAP-depleted HUVECs plated on gelatin-coated filters were subjected to a Boyden Chamber migration assay with or without VEGF (10 ng/ml). Scale bar, 100 μm. (e) Ratio of the average number of migrating cells normalized to the number of VEGF-stimulated migrating control cells from 4 independent experiments. Error bars indicate SEM (**P ≤ 0.01, ***P ≤ 0.001, One-way ANOVA, Bonferroni’s multiple comparisons post-test). (f) Control and CdGAP-depleted HUVECs were trypsinized and plated on solid Matrigel in the presence of VEGF (50 ng/ml). Scale bar, 100 μm. (g) Ratio of total capillary tube length from 3 independent experiments. Error bars indicate SEM (***P ≤ 0.001, Unpaired student’s t-test). (h) Control and CdGAP-depleted HUVECs grown as spheroids were embedded in collagen containing either VEGF (50 ng/ml) or PBS (−) as a control. Scale bar, 100 μm. (i,j) Ratio of the total length (i) and number (j) of sprouts. Error bars indicate SEM (n = 4, **P ≤ 0.01, ***P ≤ 0.001, One-way ANOVA, Bonferroni’s multiple comparisons post-test).