Abstract
Background
E‐point‐to‐septal‐separation (EPSS) and the sphericity index (SI) are echocardiographic parameters that are recommended in the ESVC‐DCM guidelines. However, SI cutoff values to diagnose dilated cardiomyopathy (DCM) have never been evaluated.
Objectives
To establish reference ranges, calculate cutoff values, and assess the clinical value of SI and EPSS to diagnose DCM in Doberman Pinschers.
Animals
One hundred seventy‐nine client‐owned Doberman Pinschers.
Methods
Three groups were formed in this prospective longitudinal study according to established Holter and echocardiographic criteria using the Simpson method of disk (SMOD): control group (97 dogs), DCM with echocardiographic changes (75 dogs) and “last normal” group (n = 7), which included dogs that developed DCM within 1.5 years, but were still normal at this time point. In a substudy, dogs with early DCM based upon SMOD values above the reference range but still normal M‐Mode measurements were selected, to evaluate if EPSS or SI were abnormal using the established cutoff values.
Results
ROC‐curve analysis determined <1.65 for the SI (sensitivity 86.8%; specificity 87.6%) and >6.5 mm for EPSS (sensitivity 100%; specificity 99.0%) as optimal cutoff values to diagnose DCM. Both parameters were significantly different between the control group and the DCM group (P < 0.001), but were not abnormal in the “last normal” group. In the substudy, EPSS was abnormal in 13/13 dogs and SI in 2/13 dogs.
Conclusions and Clinical Importance
E‐point‐to‐septal‐separation is a valuable additional parameter for the diagnosis of DCM, which can enhance diagnostic capabilities of M‐Mode and which performs similar as well as SMOD.
Keywords: Canine, Cardiology, DCM, Diagnostic tools, Dogs, Echocardiography, Heart, Occult cardiomyopathy, Ultrasound
Abbreviations
- BSA
body surface area
- DCM
dilated cardiomyopathy
- EPSS
e‐point‐to‐septal‐separation
- ESVC
European Society of Veterinary Cardiology
- IVS
interventricular septum
- LV
left ventricle
- LVEDV
left ventricular end‐diastolic volume
- LVESV
left ventricular end‐systolic volume
- LVIDd
left ventricular inner diameter in diastole
- LVIDs
left ventricular inner diameter in systole
- LVPW
left ventricular caudal wall
- SI
sphericity index
- SMOD
Simpson method of disk
- VPC
ventricular premature contraction
Dilated cardiomyopathy (DCM) is the most common acquired cardiac disease in large‐breed dogs. Especially Doberman Pinschers have a high prevalence for this disease.1, 2, 3 In this breed, an autosomal dominant pattern of inheritance is assumed.4 Typically, the progression of DCM in Doberman Pinschers can be classified into several different stages. The diseases start with disturbances at the cellular level of the myocardium, which are currently not detectable with routinely applied diagnostic methods. This phase is followed by an occult stage of the disease, during which patients do not show clinical signs. Commonly seen characteristics of the occult stage are ventricular tachyarrhythmias, whereas echocardiographic changes may or may not be appreciated during this stage of disease, ie, the dogs might have only arrhythmias or a combination of both, arrhythmias and echocardiographic changes.2, 5 Syncope or sudden death caused by ventricular tachyarrhythmias can occur.6 Diagnosis of cardiomyopathy in Doberman Pinschers is based upon the detection of ventricular arrhythmias by 24‐hour ambulatory electrocardiography (Holter) or the findings of myocardial systolic dysfunction and volume overload of the left ventricle by echocardiography, or both.2, 7
In order to provide a standardized protocol for the identification and staging of DCM, the European Society of Veterinary Cardiology (ESVC) taskforce proposed guidelines for the diagnosis of DCM using echocardiography.8 A scoring system was recommended which is based on M‐mode left ventricular dimensions, left ventricular geometry and indices of systolic function. Major and minor criteria were proposed to establish the diagnosis of DCM, including SI as major and increased mitral valve M‐mode E‐point‐to‐septal‐separation (EPSS) as minor criterion. Little is known about the SI and EPSS in veterinary medicine. However, both parameters are part of the proposed scoring system of the ESVC.
In DCM ventricular dilatation develops and the chamber becomes rounder (spherical) as the disease progresses.9 The geometrical shape, ie, the sphericity of the left ventricle (LV), can be assessed by comparing left ventricular length obtained from a right parasternal four‐chamber view to the M‐mode measurement of diastolic dimension as shown in Figure 1.10 The SI is calculated by dividing the length of the LV through the width of the LV and a value of SI <1.65 account for an increased sphericity and is considered abnormal according to the ESVC guidelines.8 Ljungvall et al evaluated left ventricular sphericity using real‐time 3‐dimensional echocardiography in dogs with myxomatous mitral valve disease. Their results proposed that the assessment of LV volume and shape could allow early detection of dogs being at risk for rapid progression into congestive heart failure.11 No study assessed the clinical value of the SI measured by conventional echocardiography as proposed in the ESVC‐DCM guidelines in veterinary medicine—there are only experimental studies using models published so far.12, 13
Figure 1.
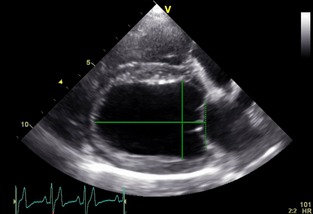
Echocardiographic right parasternal long axis. The horizontal solid line denotes the left ventricular length starting at the level of the mitral annulus (dotted vertical line) to the apex. The vertical line depicts the end‐diastolic diameter as assessed by M‐Mode measurement leveled at the tip of the opened mitral valve.
EPSS is defined by the shortest distance from the E‐point of the mitral valve (during rapid ventricular filling) to the ventricular septum as shown in Figure 2.14 It is a parameter for the evaluation of left ventricular filling and function. Kirberger et al showed that EPSS has a strong negative correlation with ejection fraction in the absence of aortic and mitral valve insufficiencies.15 Two studies have evaluated EPSS in dogs with DCM and found it to be a sensitive and specific parameter for left ventricular function.16, 17 However, in those studies, EPSS has not been compared to newer echocardiographic methods to detect DCM, such as the Simpson method of disk (SMOD), which has recently been shown to be more sensitive to diagnose early DCM in Doberman Pinschers.7
Figure 2.
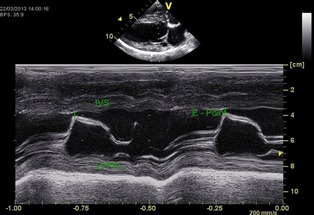
M‐Mode recording of right parasternal long axis at the level of the tip of the cranial mitral valve leaflet. The vertical line shows the distance of the maximal cranial motion (E‐Point) of the cranial mitral leaflet to the interventricular septum (IVS) during the rapid filling phase of diastole; LVPW, Left ventricular caudal wall.
No prospective longitudinal study has evaluated the SI and EPSS in Doberman Pinschers in order to diagnose DCM. Therefore, the aim of this study was to establish cutoff values for those 2 parameters and to evaluate their role as diagnostic parameters in Doberman Pinschers with dilated cardiomyopathy.
Materials and Methods
One hundred seventy‐nine client‐owned Doberman Pinschers were included into the first part of this prospective study. Written owner consent was obtained before inclusion in the study. Dogs were presented to the Clinic of Small Animal Medicine, LMU University, Munich, Germany for routine screening purposes or suspicion of cardiomyopathy, or were dogs previously diagnosed having DCM and presented for recheck examinations. This study was in accordance with the German Animal Welfare Act.
Inclusion Criteria
Control group: Dogs in the control group had to have <50 ventricular premature complexes (VPCs)/24‐hours in their Holter1 , 2 recordings.18 The end‐diastolic volume (LVEDV) and end‐systolic volume (LVESV) normalized to body surface area (BSA) were measured using Simpson's method of disk (SMOD)—LVEDV/BSA <95 mL/m² and LVESV/BSA <55 mL/m² were regarded as normal.7
DCM group: Doberman Pinschers were included into the DCM group when they had a LVESV/BSA >55 mL/m², or a LVEDV/BSA >95 mL/m², or both.7
“Last normal” group: In order to evaluate if the SI and EPSS are already abnormal at a time point before the Simpson measurements—regarded as the gold standard for diagnosis of DCM in Doberman Pinschers7—were above the reference range, a third group (“last normal”) was formed from dogs screened regularly and which developed DCM during the study period; the “last normal” examination had to be within 1.5 years before the development of DCM.
Exclusion Criteria
Dogs only showing VPCs (> 50 VPCs/24‐hours) with no echocardiographic evidence of ventricular remodeling were not included in the study. Dogs with evidence of congenital or valvular cardiac diseases or systemic diseases (including hypothyroidism) were excluded. To exclude hypothyroidism, T4 was measured and subsequent fT4 and TSH measurements, if T4 was abnormal.
Echocardiography
Echocardiography3 was performed in right and left lateral recumbency with a simultaneous ECG tracing in unsedated dogs. A 2.0/3.5 MHz transducer was used for the echocardiographic examination. Echocardiography was performed according to the recommendations of the Echocardiography Committee of the Specialty of Cardiology, American College of Veterinary Internal Medicine.19 Each variable was measured at least 3 times and the average values were used.
Measurement of SI and EPSS
SI and EPSS were measured as described above.
Study Design
The study design included 3 steps, which are shown in Figure 3.
Establishing cutoff values for SI and EPSS using the control and DCM group.
Testing these cutoff values using the “last normal” group, in order to evaluate if EPSS or SI might be already abnormal while the SMOD method is still within the reference range.
Testing the cutoff values in a M‐Mode substudy.
Figure 3.

The study design included 3 steps: (1) Establishing cutoff values for SI and EPSS using the control and DCM group. (2) Testing these cutoff values using the “last normal” group, in order to evaluate if EPSS or SI might be already abnormal whereas the SMOD method is still within the reference range. (3) Testing the cutoff values in a M‐Mode substudy
M‐Mode Substudy
As the Simpson method has previously shown to be more sensitive to detect echocardiographic changes compared to M‐Mode, the database was searched for dogs that were abnormal in the Simpson method, but in which the M‐Mode measurements were still below the published DCM cutoff values for M‐Mode. EPSS and SI were evaluated in these dogs in order to evaluate if EPSS and/or SI were already above (EPSS) or below (SI) the cutoff values established in this study and therefore abnormal. All dogs had to become abnormal in their M‐Mode measurements at a later time point (within 1.5 years) to ensure that the DCM diagnosis was correctly based upon the Simpson measurements. M‐Mode measurements for left ventricular internal dimensions at end systole (LVIDs) and end diastole (LVIDd) had to be above established reference ranges for Doberman Pinschers (LVIDd >49.0 mm, LVIDs >40.0 mm) being considered as dogs with DCM, or below those reference range to be counted as normal in M‐Mode for this part of the study, respectively.7, 20, 21, 22, 23, 24, 25
Statistical analysis was performed by commercially available statistical software.4 , 5 Descriptive statistics (mean ± standard deviation) were calculated for physiologic (age, sex, weight) and conventional echocardiographic parameters. Normal distribution of the data was evaluated using a Kolmogorov–Smirnov Analysis. Student's t‐test for unpaired samples was used to evaluate differences concerning SI and EPSS between groups. Receiver operating characteristic curves (ROC) were derived for the SI and EPSS and the area under the curve was calculated. ROC analysis was used to assess the ability of each echocardiographic parameter to discriminate healthy dogs from Doberman Pinschers with DCM, and to help estimate the optimal cutoff values that would best classify the dogs correctly. Dot diagrams were used to illustrate sensitivity and specificity of SI and EPSS to detect DCM.
Five echocardiograms were randomly selected to determine intrareader (PH) measurement variability for SI and EPSS. The same 5 echocardiograms were subjected to independent repeated analyses by a second investigator (GW) to determine interreader measurement variability. Both investigators were unaware of the results of the prior echocardiographic results. The intra‐ and interobserver coefficients of variation (CV) were calculated using a variance component analysis. The CVs were obtained by dividing the root of the variance error by the mean of the repeated measurements, times 100. Significance was defined as P < .05.
Results
For the first part of the study, 179 Doberman Pinschers (79 female and 100 male) with a mean age of 6.2 ± 2.8 years and a mean body mass of 35.8 ± 5.5 kg were included into the study. Ninety‐seven of those dogs (53 female, 44 male; 5.4 ± 2.6 years; 35.3 ± 5.3 kg) were considered as being healthy according to the criteria stated above; 75 dogs had DCM (25 female, 50 male; 7.4 ± 2.7 years; 36.1 ± 5.7 kg) and 7 dogs were assigned to the “last normal” group (1 female, 7 male; 5.1 ± 2.2 years; 39.5 ± 1.8 kg). Echocardiographic values are shown in Table 1.
Table 1.
Echocardiographic values of the healthy, DCM and “last normal” groups. LVEDV/BSA and LVESV/BSA denoted the left ventricular diastolic and systolic volume normalized to the body surface area—all values given in milliliters per square meter. LVIDd and LVIDs show the left ventricular inner diameter in diastole and systole with values given in centimeters. EPSS depicts the E‐point‐to‐septal‐separation in centimeters; SI denotes an index without numeric dimensions
| Control | DCM | Last Normal | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Range | Mean | SD | Range | Mean | SD | Range | |
| LVEDV/BSA, mL/m² | 76.4 | 9.0 | 55.7–96.1 | 138.0 | 37.7 | 96.8–289.0 | 78.4 | 7.8 | 65.6–88.0 |
| LVESV/BSA, mL/m² | 37.7 | 7.4 | 15.8–54.0 | 93.8 | 36.5 | 56.0–217.0 | 39.5 | 7.74 | 30.2–48.8 |
| LVIDd, mm | 39.9 | 3.1 | 33.6–47.2 | 54.7 | 7.2 | 43.0–78.9 | 42.2 | 3.3 | 38.0–48.0 |
| LVIDs, mm | 28.9 | 3.6 | 11.7–39.1 | 45.6 | 8.0 | 31.7–72.1 | 29.6 | 2.2 | 28.0–33.4 |
| EPSS, mm | 4.66 | 0.69 | 2.89–7.19 | 12.10 | 3.21 | 7.19–20.2 | 4.50 | 0.88 | 3.07–5.56 |
| SI | 1.86 | 0.17 | 1.42–2.26 | 1.44 | 0.16 | 1.11–1.78 | 1.86 | 0.17 | 1.69–2.05 |
Of the dogs in the DCM group, 7 animals were decompensated and had evidence of pulmonary edema on radiographs, 4 of the remaining dogs had a history of syncope. The remaining dogs did not have clinical symptoms. The dogs in the DCM group received Pimobendan in 54 cases, Sotalol in 23 cases, and Amiodarone in 14 cases. All dogs with pulmonary edema received furosemide, an ACE‐inhibitor and Pimobendan.
Measurements of SI and EPSS were Normally Distributed within the Groups
The SI (1.44 ± 0.16) was significantly smaller (P < .001) in the DCM group compared to the healthy Doberman pinscher group (1.86 ± 0.17). ROC analysis determined that the optimal cutoff value was <1.65. Using this cutoff value the sensitivity was 86.8% and specificity was 87.6% to differentiate dogs with DCM form the control group (Fig 4). Of the dogs in the “last normal” group, 0/7 had an SI < 1.65.
Figure 4.
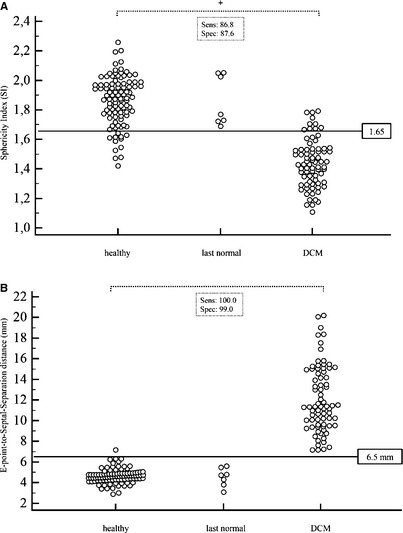
Dot diagram of the SI (A) and EPSS (B) on the y‐axis and the different groups on the x‐axis. The horizontal line denotes the estimated cutoff value to distinguish healthy dogs from dogs with DCM. There was a significant difference between DCM and control group (P < .001). Sensitivity and specificity are displayed.
EPSS was significantly higher in the DCM group (12.1 ± 3.21) compared to the control group (4.66 ± 0.69, P < .001). ROC analysis revealed an EPSS >6.5 mm as the value with the highest sensitivity (100%) and specificity (99%) to detect DCM. None of the dogs in the “last normal” group had an EPSS >6.5 mm (Fig 4). Therefore, neither EPSS nor SI was abnormal before changes detected with the SMOD method, which is considered the gold standard to detect echocardiographic changes as mentioned earlier.
For the evaluation, if EPSS, or SI, or both would be abnormal using the herein established cutoff values in dogs having M‐Mode measurements below the published DCM cutoff values, but with Simpson measurements consistent with DCM, EPSS and SI were measured in a substudy. Thirteen dogs were found in the database to fulfill these inclusion criteria. All dogs developed typical M‐Mode measurements consistent with DCM within 1.5 years. All of the 13 dogs had an EPSS >6.5 mm, whereas only 2/13 dogs had an SI below the cutoff value <1.65.
Intraobserver coefficients of variation for SI and EPSS were 1.53 and 2.29%, respectively. Interobserver coefficients of variation were 3.53% for the SI and 4.83% for EPSS.
Discussion
The results of this study revealed that the SI using a cutoff value of 1.65 had a good sensitivity (86.8%) and specificity (87.6%) to differentiate dogs with DCM from control dogs. The best (calculated) cutoff value in this study was 1.65, which is the same cutoff value suggested by the ESVC DCM guidelines, although this cutoff value was published without an evaluation study. However, there was a considerable overlap between the control and DCM group and as neither sensitivity nor specificity is very high, the question arises, if it is necessary or useful to perform this parameter as an additional diagnostic tool. Adding this value to the standard echocardiographic examination does not prolong the examination considerably, as only the length of the left ventricle (LV) needs to be measured in addition to the M‐Mode examination. If an SMOD measurement is done routinely, the LV length is already part of that measurement and no additional measurements need to be performed. The SI aims to detect a geometrical change of the LV when the dog develops DCM, ie, that the heart becomes rounder. Therefore, the index includes the diastolic LV chamber diameter, and, as a matter of fact, the sphericity can only be changed, if there is an LV volume overload already present. As the volume of the LV is already assessed using the SMOD measurements, it is not surprising that the SI is inferior to the SMOD measurements. In humans, the SMOD is the preferred echocardiographic method to perform left ventricular volume measurements.26 Recently, this method has been shown to be more sensitive than to M‐Mode to diagnose early echocardiographic changes in Doberman Pinschers and has been therefore recommended as new echocardiographic gold standard to detect DCM in Doberman Pinschers.7 It was surprising to see how well EPSS performed compared with SMOD measurements in this study—having a sensitivity of 100% and a specificity of 99% to detect Doberman Pinschers with DCM using a cutoff value >6.5 mm. The “last normal” group was selected in order to evaluate if this parameter might actually be better compared to the Simpson method, to detect even earlier echocardiographic changes, but neither SI nor EPSS was abnormal in this group of dogs. This was expected, as the Simpson method adjusts to geometrical changes of the LV. M‐Mode does not necessarily detect early geometrical changes. It has been shown that M‐Mode is less sensitive to evaluate early echocardiographic changes in dogs with DCM, and thus, a substudy was performed using dogs that were abnormal in their SMOD‐derived ventricular volumes, but still had normal M‐Mode measurements. Whereas SI was abnormal in only 2/13 dogs in this group, all EPSS measurements were already above the cutoff value. Therefore, EPSS seems to be a valuable parameter, especially if M‐Mode instead of Simpson measurements is used, as EPSS might be earlier abnormal than M‐Mode.
EPSS has already been evaluated in Doberman Pinschers in 1986.17 EPSS was the most sensitive and specific criterion for early cardiomyopathy—showing no overlap between healthy and dogs with advanced DCM. A mean value of 4.8 mm ± 1.51 (range 3–7) was proposed and later a cutoff value of >9 mm was suggested to diagnose DCM by the same authors.5 Those results are in agreement with this study, concerning the fact that EPSS appears to be more sensitive than M‐Mode, at least when the published references for M‐Mode are used, which might be too high. However, there is a discrepancy of the cutoff values for EPSS suggested earlier17 and this study. This might be explained by the fact that in this study the Simpson method was used as gold standard, which is more sensitive to detect early changes—this explains why EPSS in this study was 12.1 ± 3.2 and in the previous study 17.2 ± 8.59.17 The higher EPSS values in the Calvert study in the normal group might be explained by the fact that dogs were included in the control group, which had M‐Mode values (LVIDd: control group range 41–55 mm), which are considered to be clearly too high today.7 EPSS has been also evaluated in Irish Wolfhound suggesting to be a sensitive parameter to detect DCM.16
No distinct EPSS value has been published by the ESVC taskforce8 for the diagnosis of DCM. Because of the fact that neither breed, age, sex, body mass nor heart rate showed a significant correlation with EPSS, an overall value can be proposed.15 But still care must be taken when regional or general hypertrophy is seen—this can cause a decrease in EPSS because of restricted motion of the mitral valve.27 In the presence of mitral or aortic regurgitation, EPSS as a parameter of systolic function cannot be assessed objectively anymore because of a change in hemodynamics and cardiac contractility.28
A limitation of the study is the natural progression of the disease. As the occult phase of DCM in Doberman Pinschers can last for several years and might be difficult to detect in the early stage, we cannot exclude that there were some dogs in the control group that might have developed DCM at a later time point or already had very early cardiac changes that were not detectable. This could have led to a higher EPSS or lower SI in the control group, but as the control group was quite large, the effect is most likely not relevant. Another limitation is the potential effect of medical treatment on the SI and EPSS measurements, as most dogs in the DCM group received Pimobendan and about half of the dogs received antiarrhythmic drugs. The cardiac medication and its potential hemodynamic effects could have had an effect on preload, afterload, and systolic function, and therefore theoretically on SI and EPSS. However, SI and EPSS were significantly different despite treatment.
In conclusion, EPSS (>6.5 mm) is a valuable parameter, which is almost as good, but not better than the Simpson method for the diagnosis of DCM in Doberman Pinschers. EPSS seems to be especially valuable as an additional echocardiographic parameter if M‐Mode is used or in addition to the Simpson method. The SI does not appear to be more sensitive than the M‐Mode and is inferior to the Simpson measurements.
Acknowledgment
Conflict of Interest Declaration: The authors disclose no conflict of interest.
Footnotes
Custo tera. Arcon Systems GmbH, Starnberg, Germany.
Amedtech ECGpro Holter Software, EP 810 digital Recorder. Medizintechnik Aue GmbH, Aue, Germany
Vivid 7 dimensions. General Electric Medical System, Waukesha, WI
PASW Statistics, Version 18.0, IBM Corporation, Armonk, NY
MedCalc, Version 12.5, Ostend, Belgium
References
- 1. Tidholm A, Jonsson L. A retrospective study of canine dilated cardiomyopathy (189 cases). J Am Anim Hosp Assoc 1997;33:544–550. [DOI] [PubMed] [Google Scholar]
- 2. Wess G, Schulze A, Butz V, et al. Prevalence of dilated cardiomyopathy in Doberman Pinschers in various age groups. J Vet Intern Med 2010;24:533–538. [DOI] [PubMed] [Google Scholar]
- 3. Summerfield NJ, Boswood A, O'Grady MR, et al. Efficacy of pimobendan in the prevention of congestive heart failure or sudden death in Doberman Pinschers with preclinical dilated cardiomyopathy (the PROTECT Study). J Vet Intern Med 2012;26:1337–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Meurs KM, Fox PR, Norgard M, et al. A prospective genetic evaluation of familial dilated cardiomyopathy in the Doberman pinscher. J Vet Intern Med 2007;21:1016–1020. [DOI] [PubMed] [Google Scholar]
- 5. Calvert C, Meurs KM. CVT update: Doberman Pinschers occult cardiomyopathy In: Bonagura J, ed. Kirk's Current Veterinary Therapy. Philadelphia, PA: WB Saunders Company; 2000:800–803. [Google Scholar]
- 6. Calvert C, Hall G, Jacobs G, Pickus C. Clinical and pathologic findings in Doberman Pinschers with occult cardiomyopathy that died suddenly or developed congestive heart failure. J Am Vet Med Assoc 1997;210:505–511. [PubMed] [Google Scholar]
- 7. Wess G, Maurer J, Simak J, et al. Use of Simpson's method of disc to detect early echocardiographic changes in Doberman Pinschers with dilated cardiomyopathy. J Vet Intern Med 2010;24:1069–1076. [DOI] [PubMed] [Google Scholar]
- 8. Dukes‐McEwan J, Borgarelli M, Tidholm A, et al. Proposed guidelines for the diagnosis of canine idiopathic dilated cardiomyopathy. J Vet Cardiol 2003;5:7–19. [DOI] [PubMed] [Google Scholar]
- 9. Lee BH, Dukes‐McEwan J, French AT, et al. Evaluation of a novel Doppler index of combined systolic and diastolic myocardial performance in Newfoundland dogs with familial prevalence of dilated cardiomyopathy. Vet Radiol Ultrasound 2002;43:154–165. [DOI] [PubMed] [Google Scholar]
- 10. Boon JA. Myocardial disease In: Boon JA, ed. Veterinary Echocardiography. West Sussex, UK: Wiley‐Blackwell; 2011:388–390. [Google Scholar]
- 11. Ljungvall I, Hoglund K, Carnabuci C, et al. Assessment of global and regional left ventricular volume and shape by real‐time 3‐dimensional echocardiography in dogs with myxomatous mitral valve disease. J Vet Intern Med 2011;25:1036–1043. [DOI] [PubMed] [Google Scholar]
- 12. Kono T, Sabbah HN, Rosman H, et al. Left ventricular shape is the primary determinant of functional mitral regurgitation in heart failure. J Am Coll Cardiol 1992;20:1594–1598. [DOI] [PubMed] [Google Scholar]
- 13. Sabbah HN, Kono T, Rosman H, et al. Left ventricular shape: A factor in the etiology of functional mitral regurgitation in heart failure. Am Heart J 1992;123:961–966. [DOI] [PubMed] [Google Scholar]
- 14. Boon JA. Evaluation of size, function, and hemodynamics In: Boon JA, ed. Veterinary Echocardiography. West Sussex, UK: Wiley‐Blackwell; 2011:182–183. [Google Scholar]
- 15. Kirberger RM. Mitral valve E point to ventricular septal separation in the dog. J S Afr Vet Assoc 1991;62:163–166. [PubMed] [Google Scholar]
- 16. Vollmar AC. Use of echocardiography in the diagnosis of dilated cardiomyopathy in Irish Wolfhounds. J Am Anim Hosp Assoc 1999;35:279–283. [DOI] [PubMed] [Google Scholar]
- 17. Calvert CA, Brown J. Use of M‐mode echocardiography in the diagnosis of congestive cardiomyopathy in Doberman Pinschers. J Am Vet Med Assoc 1986;189:293–297. [PubMed] [Google Scholar]
- 18. Wess G, Schulze A, Geraghty N, et al. Ability of a 5‐minute electrocardiography (ECG) for predicting arrhythmias in Doberman Pinschers with cardiomyopathy in comparison with a 24‐hour ambulatory ECG. J Vet Intern Med 2010;24:367–371. [DOI] [PubMed] [Google Scholar]
- 19. Thomas WP, Gaber CE, Jacobs GJ, et al. Recommendations for standards in transthoracic two‐dimensional echocardiography in the dog and cat. Echocardiography Committee of the Specialty of Cardiology, American College of Veterinary Internal Medicine. J Vet Intern Med 1993;7:247–252. [DOI] [PubMed] [Google Scholar]
- 20. Calvert CA, Brown J. Influence of antiarrhythmia therapy on survival times of 19 clinically healthy Doberman Pinschers with dilated cardiomyopathy that experienced syncope, ventricular tachycardia, and sudden death (1985‐1998). J Am Anim Hosp Assoc 2004;40:24–28. [DOI] [PubMed] [Google Scholar]
- 21. Calvert CA, Jacobs G, Pickus CW, et al. Results of ambulatory electrocardiography in overtly healthy Doberman Pinschers with echocardiographic abnormalities. J Am Vet Med Assoc 2000;217:1328–1332. [DOI] [PubMed] [Google Scholar]
- 22. O'Grady MR, O'Sullivan ML. Dilated cardiomyopathy: An update. Vet Clin North Am Small Anim Pract 2004;34:1187–1207. [DOI] [PubMed] [Google Scholar]
- 23. O'Grady MR, Horne R. Outcome of 103 asymptomatic Doberman Pinschers: Incidence of dilated cardiomyopathy in a longitudinal study. J Vet Intern Med 1995;9:199. [Google Scholar]
- 24. O'Grady MR, Horne R. Occult dilated cardiomyopathy: An echocardiographic and electrocardiographic study of 193 asymptomatic Doberman Pinschers. J Vet Intern Med 1992;2:112. [Google Scholar]
- 25. O'Grady MR, Horne R. Echocardiographic findings in 51 normal Doberman Pinschers. J Vet Intern Med 1995;2:202. [Google Scholar]
- 26. Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: A report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 2005;18:1440–1463. [DOI] [PubMed] [Google Scholar]
- 27. Ginzton LE, Kulick D. Mitral valve E‐point septal separation as an indicator of ejection fraction in patients with reversed septal motion. Chest 1985;88:429–431. [DOI] [PubMed] [Google Scholar]
- 28. Boon JA. Acquired valvular disease In: Boon JA, ed. Veterinary Echocardiography. West Sussex, UK: Wiley‐Blackwell; 2011:267–319. [Google Scholar]


