Abstract
Background
An intravenous (IV) overdose of pentobarbital sodium is the most commonly used method of euthanasia in veterinary medicine. However, this compound is not available in many countries or rural areas resulting in usage of alternative methods such as intrathecal lidocaine administration after IV anesthesia. Its safety and efficacy as a method of euthanasia have not been investigated in the horse.
Hypothesis/Objectives
To investigate changes in mean arterial blood pressure and electrical activity of the cerebral cortex, brainstem, and heart during intrathecal administration of lidocaine. Our hypothesis was that intrathecal lidocaine affects the cerebral cortex and brainstem before affecting cardiovascular function.
Animals
Eleven horses requiring euthanasia for medical reasons.
Methods
Prospective observational study. Horses were anesthetized with xylazine, midazolam, and ketamine; and instrumented for recording of electroencephalogram (EEG), electrooculogram (EOG), brainstem auditory evoked response (BAER), and electrocardiogram (ECG). Physical and neurological (brainstem reflexes) variables were monitored. Mean arterial blood pressure was recorded throughout the study.
Results
Loss of cerebro‐cortical electrical activity occurred up to 226 seconds after the end of the infusion of lidocaine solution. Cessation of brainstem function as evidenced by a lack of brainstem reflexes and disappearance of BAER occurred subsequently. Undetectable heart sounds, nonpalpable arterial pulse, and extremely low mean arterial blood pressure supported cardiac death; a recordable ECG was the last variable to disappear after the infusion (300–1,279 seconds).
Conclusions and Clinical Importance
Intrathecal administration of lidocaine is an effective alternative method of euthanasia in anesthetized horses, during which brain death occurs before cardiac death.
Keywords: Brainstem auditory evoked response, Death, Electroencephalogram, Equine
Abbreviations
- BAER
brainstem auditory evoked response
- ECG
electrocardiogram
- EEG
electroencephalogram
- EMG
electromyography
- EOG
electrooculogram
Ending an animal's life in a painless and minimally distressful way is the basis of euthanasia. The American Veterinary Medical Association (AVMA) has published guidelines for the euthanasia of animals.1 These guidelines acknowledge that euthanasia is a process involving professional judgement as to when to consider euthanasia and the events before (eg, sedation), during (method and agents), and after (animal disposal) humane death.1 The main method of euthanasia in horses consists of the use of an intravenous (IV) overdose of barbiturates or barbituric acid derivatives with or without prior sedation with α‐adrenergic receptor agonists in conjunction with opioid agonists.1 Acceptable methods include physical and adjunctive methods.1 Physical methods consist of the use of a penetrating captive bolt and gunshot.1 Adjunctive methods are those in which IV anesthesia is used followed by an adjunctive IV method.1 For example, euthanasia with potassium chloride to induce cardiac arrest is an adjunctive method only acceptable when a saturated solution is given IV or intracardially under a deep plane of general anesthesia.1 The American Association of Equine Practitioners guidelines for euthanasia in horses are similar to those of AVMA.2
Although these methods of euthanasia are available and regarded as standard practice in the United States and other countries, in many other parts of the world alternatives for euthanasia are more limited.1 Concentrated barbiturate solutions often are not available, illegal, or prohibitively expensive.1 Furthermore, in remote rural communities rendering or burial facilities are unavailable, and the carcasses of horses euthanized with barbiturates are considered toxic1 and might be eaten by scavengers, carnivores, and even humans. Gunshot to the brain can be problematic because of the legalities of carrying and using firearms in many countries. Gunshot also puts the veterinarian and bystanders at risk from accidental discharge or ricochet.1 Penetrating captive bolt devices are expensive, of limited use, and usually unavailable. Intravenous or intracardiac potassium chloride requires a large volume to achieve cardiac arrest at a dosage of 2 mmol/kg.1 Furthermore, its preparation is time consuming, and intracardiac administration requires training and can be technically challenging. Other methods of euthanasia have been used when concentrated barbiturate solution is not available. Anecdotally, 2% lidocaine hydrochloride has been administered into the intrathecal space with the horse under IV anesthesia as an alternative method of euthanasia.2 Lidocaine hydrochloride is inexpensive and may be more readily available in some countries. Additionally, the supplies used for this procedure have a variety of other uses for routine and emergency veterinary medicine in the field. These considerations become important when working in remote communities where the efficient use of packing space is required for transportation. However, the lack of availability of drugs needed for the induction of anesthesia before intrathecal administration in some parts of the world might defeat the purpose of using lidocaine as an alternative method. Furthermore, intrathecal infusion of lidocaine could be technically challenging without proper training.
A recent study of cerebral and brainstem electrophysiologic activity during euthanasia with pentobarbital sodium in horses demonstrated that this method of euthanasia is effective and rapid.3 In that study, respiratory arrest occurred during the infusion, followed by loss of cerebro‐cortical activity within 52 seconds after completion of the infusion (supported by lack of awareness), and then loss of auscultable heart sounds and extremely low mean arterial blood pressure (lack of cardiac output), loss of brainstem activity (supportive of brainstem death), and lastly lack of ECG activity.3 The efficacy of intrathecal lidocaine hydrochloride under IV anesthesia as a method to induce loss of consciousness, cardiac and respiratory arrest is unknown. This approach might represent a major welfare concern that should be investigated because this method is already in practice without previous objective assessment.2 Therefore, the purpose of the study was to investigate whether changes in respiratory, cardiovascular, and neurologic variables in support of respiratory and cardiac arrest and brain death occur with intrathecal administration of lidocaine hydrochloride. Our hypothesis was that intrathecal lidocaine affects cerebro‐cortical and brainstem activity before cardiovascular function.
Materials and Methods
Animals and Study Design
This observational prospective study included 11 horses for which euthanasia was elected based on published guidelines during a study period from September to December 2014.1 Reasons for euthanasia included poor quality of life, intractable pain, or chronic progressive debilitating or incapacitating disease with a poor prognosis. Horses were sourced from a research herd from the University of California at Davis. The study was approved by the institutional animal care and use committee.
Pre‐Euthanasia Protocol: Intravenous Anesthesia
All horses had an IV catheter placed in the jugular vein for the administration of sedatives and injectable anesthetics. Intravenous anesthesia consisted of xylazine hydrochloride at a dosage of 1 mg/kg followed 5 minutes later by midazolam hydrochloride at 0.05 mg/kg and ketamine hydrochloride at 2.2 mg/kg. Xylazine (0.3 mg/kg) and ketamine (0.7 mg/kg) were repeated as needed during instrumentation that included catheterization of the facial artery for monitoring arterial blood pressure and needle electrodes for electrophysiologic studies.
Physical and Neurological Variables
Physical variables included audible heart rate and rhythm, and the presence and quality of the arterial pulse. The neurologic variables consisted of presence or absence of brainstem reflexes such as direct pupillary light, corneal, and palpebral reflexes. The subcortical dazzle reflex also was monitored. These variables were monitored as follows: (1) before receiving any IV medication, (2) baseline once anesthetized, (3) after instrumentation (arterial catheterization, electroencephalogram [EEG], electrooculogram [EOG], electrocardiogram [ECG], and brainstem auditory evoked response [BAER]), (4) immediately after centesis and withdrawal of CSF from the atlanto‐occipital cistern, (5) within 20 seconds after initiation of the infusion, (6) immediately after the end of the infusion, and (7) every 30 seconds thereafter until these variables were undetectable. Personnel assistance was used for monitoring physical (1st assistant) and neurologic (2nd assistant) variables. Mean arterial blood pressure (MAP) was recorded continuously.
Electrophysiologic Examination
The examination consisted of EEG, EOG, BAER, and ECG performed as described elsewhere.4, 5 The equipment used for EEG, EOG, and ECG was a wireless (telemetry) digital EEG system3 with integrated video monitoring. Instrumentation for these procedures has been described elsewhere.4 Needle electrodes were placed SC in the scalp of the horse for the recording of EEG.4 Baseline recordings were performed before euthanasia, and continuous recordings were made throughout the procedure in all horses.
An evoked potential system4 was used for recording BAER. One set of baseline recordings (signal average of 200 responses using 2 derivations [vertex to mastoid, vertex to C2]5 run simultaneously) with a single duplicate recorded for each ear were made before euthanasia. Immediately after the initial BAER recording, infusion of intrathecal lidocaine began and additional recordings were obtained. Each complete recording took 90 seconds. These were repeated continuously until BAER was absent (ie, no peaks could be detected). The sound intensity applied to the ear under examination was 90 dB normal hearing level (nHL) with a masking sound of 60 dB nHL applied to the contralateral ear.5 Peaks were labeled from I to V; these were consistent with the presence of auditory function.5
Euthanasia Protocol
An area over the atlanto‐occipital space was clipped and cleaned aseptically with betadine solution for cerebrospinal fluid (CSF) centesis as described previously.6 An 18‐gauge 10.62‐cm (3 inches) needle was used for the collection of 60 mL of CSF (only 30 mL in an Arabian colt) and administration of 2% lidocaine hydrochloride at a dosage of 4 mg/kg administered within 30 seconds. The collection volume of 60 mL of CSF was selected based on current anecdotal practices and the convenience of widely available 60 mL syringes.2 At the end of the infusion, the needle was removed and physical, neurologic, and electrophysiologic variables recorded.
Statistical Analysis
Data were summarized as mean, standard deviation (SD), median and range values.
Results
Eleven horses of Thoroughbred (n = 10) and Arabian (n = 1) breeds were included in the study. There were 4 males (castrated = 3, intact = 1), and 7 females. The mean age was 13.2 years (median, 10; range, 10 months to 24 years). Seven horses had multiple chronic orthopedic disease, 2 had neurologic disease (epilepsy = 1, progressive multifocal spinal cord disease = 1), and 2 had chronic progressive systemic disease (metastatic melanoma = 1, weight loss = 1).
Respiratory and Cardiovascular Variables
Heart rate increased during and immediately after the administration of lidocaine. Visible and audible breaths were not evident 120 seconds (2 minutes) after the end of the infusion (Table 1). Heart sounds were not audible 614 seconds (10.2 minutes) after the end of the infusion (Table 1). Mean arterial pressure decreased from a mean of 121 mmHg before the infusion to 42 mmHg 5 minutes after the end of the infusion, and decreased to its lowest point within 688 seconds (11.5 minutes; mean, 35 mmHg) after the infusion (Table 1). Despite undetectable heart sounds and the absence of a palpable arterial pulse, ECG monitoring showed ongoing activity until a mean time of 746.6 seconds (median 705; range, 300–1,279 seconds [up to 21.3 minutes]) from termination of the infusion (Table 1). Before complete loss of ECG activity in all horses, ECG waves became irregular in shape, size, and rhythm.
Table 1.
Cardiovascular and neurologic variables. Values for intrathecal (IT) lidocaine and intravenous (IV) barbiturates infusion are presented. Times at which respiration, heart sounds (HS), ECG, EEG, brainstem reflexes (BS), and BAER were undetectable after completion of the infusion. Time is in seconds (sec) for all variables. Negative numbers represent time of undetectable variable before completion of the infusion. The same variables are shown for the barbiturate study (n = 15 horses, except if marked* = 8 horses).3 NA = Not applicable because of being undetectable early during the infusion
| Protocol | Respiratory | Cardiovascular | Neurologic | ||||
|---|---|---|---|---|---|---|---|
| Undetectable (sec) | Inaudible HS (sec) | Flat ECG (sec) | Flat EEG (sec) | No BS (sec) | Flat BAER (sec) | ||
| IT Lidocaine (N = 11) | Mean (SD) | 33.9 (36.2) | 373.7 (120.5) | 746.6 (355.3) | 144.7 (64.1) | 170.5 (96.4) | 329.5 (86.4) |
| Median (range) | 25 (−30 to 120) | 400 (221 to 614) | 705 (300 to 1,279) | 131 (44 to 226) | 207 (44 to 284) | 316 (172 to 444) | |
| IV Barbiturate (N = 15) | Mean (SD) | NA | 43.2 (12.1) | 559.1 (217.9) | 23.7 (21.3) | 81.1 (39) | 122.6* (69.6) |
| Median (range) | NA | 38 (25 to 60) | 501 (330 to 979) | 18 (−90 to 52) | 80 (36 to 169) | 88* (73 to 261) | |
Neurologic Variables
All horses had intact brainstem reflexes before intrathecal infusion of lidocaine. The EEG recordings were of continuous activity before and during the infusion (Fig 1A). All 5 BAER peaks were readily identified on baseline recordings. Lack of detection of EEG (a continuous isoelectric pattern) occurred at a mean time of 144.7 seconds (median, 131; range, 44–226 seconds) after the end of the infusion (Table 1). Lack of brainstem reflexes occurred a mean of 170.5 seconds (median, 207; range, 44–284 seconds) after the end of the infusion. A breath‐like movement (perceived as an agonal breath) concurrent with undetectable brainstem reflexes was observed in 2 horses (Table 1, Fig 1B). Decreased amplitudes of all BAER peaks were noted seconds after termination of the infusion, with peak I being the last to disappear (Fig 2). Loss of a detectable BAER was seen at a mean time of 329.5 seconds (median, 316; range, 172–444 seconds) after completion of the infusion (Fig 2). At the time of undetectable BAER, the EEG was isoelectric (Fig 3).
Figure 1.
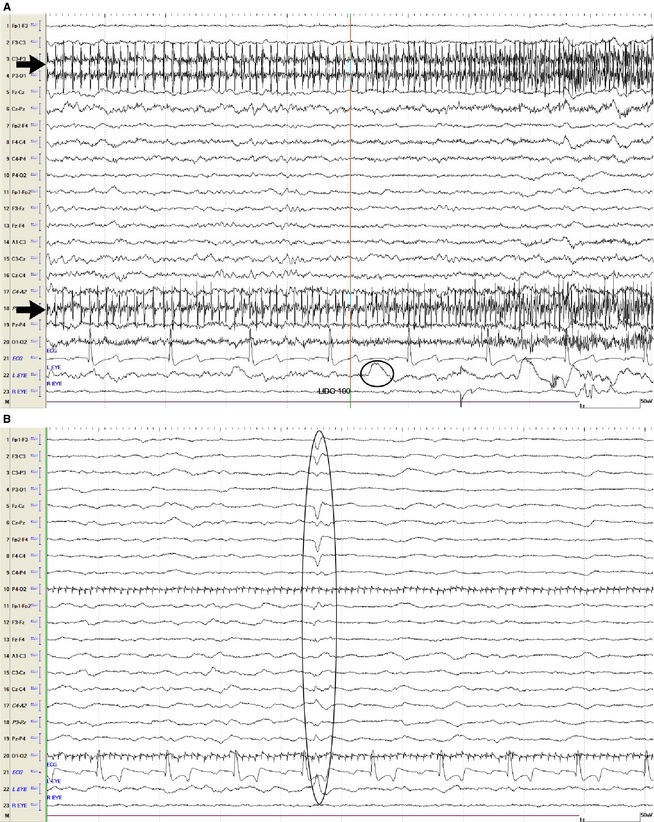
(A) This EEG recording is depicting the end of the intrathecal infusion with 100 mL of lidocaine (line labeled as lido 100 marks the end of the infusion). Muscle (black arrows) and eye (black oval) movement can be seen. (B) EEG becoming isoelectric. Note the artifact of an agonal breath (oval) concurrently with loss of brainstem reflexes. Muscle (motor unit) artifact is apparent. For all figures showing electroencephalogram: Even numbers = right side, odd numbers = left side, z = midline. Fp = frontopolar, F = frontal, C = central, P = parietal, O = occipital, A = aural, EOG: right eye or left eye, ECG. Calibration bar shown is for EEG and EOG = 1 second (second), 50 µV (microvolts). Calibration bar for ECG is not shown.
Figure 2.
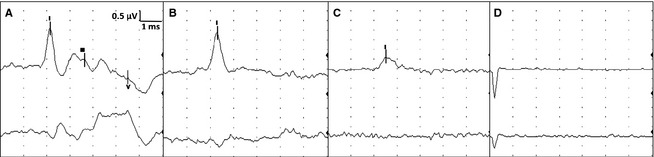
Brainstem auditory evoked response (BAER). For all figures: Top tracing is vertex to mastoid (V‐M) and bottom tracing is vertex to C2 (V‐C2) recorded simultaneously. This figure represents stimulation of the left ear only. Calibration indicated for all figures (0.5 µV = microvolts, 1 ms = 1 millisecond per division). (A) Baseline BAER before euthanasia infusion. (B) BAER done at the time of absent brainstem reflexes. (C) BAER just before becoming absent. Note only wave I is visible on V‐M but with decreased amplitude and slower latency than baseline. (D) Absent BAER.
Figure 3.
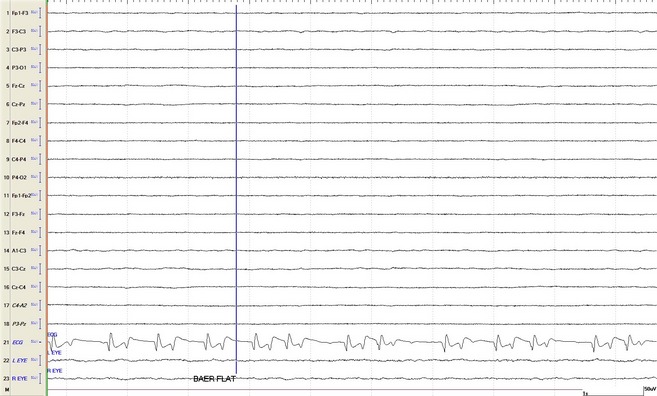
Isoelectric EEG pattern recorded at the time when brainstem reflexes were absent. Brainstem auditory evoked response (BAER) loss is depicted by vertical line (flat BAER). Note irregular heart rate and altered complex morphology. For abbreviations see Fig 1.
Discussion
This study showed that euthanasia with intrathecal lidocaine hydrochloride administration under IV anesthesia is an effective method to end life. However, this technique results in longer time to respiratory, cerebral, brainstem, and cardiovascular arrest when compared to the most common practice of an overdose of IV barbiturates.3 The sequential order of events in this study consisted of loss of respiration, cerebro‐cortical activity, brainstem (first reflexes, then BAER), and cardiovascular activity (heart sounds, and lastly ECG). These findings are in contrast to euthanasia with barbiturates during which the sequence of events consisted of loss of respiration, cerebro‐cortical activity, heart sounds, brainstem (first reflexes, then BAER), and lastly ECG activity.3
Intrathecal lidocaine administration has been used widely in human medicine for spinal anesthesia because of its local anesthetic effects as a sodium channel blocker.7, 8 Other mechanisms of action affecting neuronal transmission include the inhibition of G‐protein coupled receptor and N‐methyl‐D‐aspartate receptors.9 Therefore, a possible explanation for the observed sequence of events during euthanasia with intrathecal lidocaine could have been from a direct anesthetic effect on neural structures associated with the route of administration. Furthermore, the dosage used here was substantially higher than any epidural dosage used in equine practice (0.35–0.4 mg/kg versus 4 mg/kg in this study).10 Although lidocaine is known to affect the cardiovascular system,11 the route used here might have prolonged the time it took to affect this system. Lidocaine delivered directly into the subarachnoid space close to the brain could have resulted in neural anesthesia initially, as demonstrated by the loss of cerebro‐cortical activity and brainstem function relative to the cardiovascular variables, whereas IV barbiturates distribute rapidly to the cardiovascular system because of the route of administration with subsequent rapid distribution to the brain.3
Similar to the barbiturate study, loss of respiratory variables (observable and auscultable breaths) occurred first.3 Loss of respiratory variables occurred while lidocaine was being administered and up to 2 minutes after the end of the infusion, whereas undetectable breaths occurred in all horses by the end of barbiturate infusion.3 Similarly, a few breath‐like movements occurred at a time when EEG activity and brainstem reflexes were absent, and therefore were considered reflexive (agonal breaths and not true breaths).3
Absence of detectable cerebro‐cortical electrical activity occurred up to 3.8 minutes after the end of lidocaine infusion. This lack of EEG activity appeared to be irreversible based on continuous recording for several minutes with no recovery of EEG activity. In contrast, lack of EEG activity occurred while administering an overdose of barbiturates IV up to 52 seconds after the end of the infusion.3 Electroencephalography primarily represents cerebro‐cortical activity and an isoelectric pattern (absent EEG) has been used to aid in the determination of brain death in human medicine.12 Also, an isoelectric recording is consistent with a lack of consciousness, and therefore a lack of awareness of the events related to euthanasia. As is the case with other diagnostic electrophysiologic techniques, numerous factors such as states of disease, body temperature, patient movement, medications, and electrical interference can impact the quality of the recordings obtained and make interpretation difficult.13, 14 With 1 exception, none of the 11 horses had an illness that would have influenced EEG activity. However, the horse with epilepsy had continuous EEG activity on the baseline recording and this activity did not interfere with the identification of the onset of the isoelectric pattern. Movement artifacts were avoided in this study because the horses were anesthetized. The chemical agents used for IV anesthesia in this study (xylazine hydrochloride, ketamine hydrochloride, and midazolam) have not been reported to induce burst suppression or isoelectric patterns.4 Interference from electrical and medical equipment was unlikely because the only other machine present during the study was a device to measure arterial blood pressure, in addition to the EEG and BAER equipment.
Brainstem function was lost after the loss of cerebro‐cortical activity based on absent brainstem reflexes and BAER. Brainstem reflexes were undetectable up to 4.7 minutes after the end of the lidocaine infusion, and occurred before loss of BAER. Agonal breaths (Fig 1B) were observed in 1 horse and happened concurrently with the loss of brainstem reflexes. In the euthanasia study with barbiturates, brainstem reflexes were lost up to 2.8 minutes from the end of the infusion.3
Brainstem auditory evoked response assesses the auditory pathway from the cochlear nerve to the caudal and cranial brainstem.15 Therefore, BAER could be used to evaluate the presence or absence of brainstem function and serve as a diagnostic aid in the determination of brain death.15 Part of the criteria of brain death in humans includes the absence of waves after wave I on a BAER.16 All 11 of these horses had normal baseline BAERs before the infusion of lidocaine. All BAER peaks were present in all horses and considered to be within published reference ranges.17, 18 Similar to the euthanasia study with IV barbiturates, the amplitude of all peaks decreased and interpeak intervals increased within seconds after termination of lidocaine infusion.3 Similarly, loss of peaks II to V (brainstem) occurred first, and peak I was the last wave to become undetectable (Fig 2). Complete absence of BAER in support of brain death in the absence of brainstem disease in these 11 horses was observed from 2.9 to 7.4 minutes after the end of the infusion of lidocaine. Subsequent BAER testing up to 15 minutes after the first observation of absent BAER showed no return of BAER. In contrast, barbiturate infusion resulted in absent BAER from 1.2 to 4.4 minutes after the end of the infusion (Table 1).3 Despite high doses of barbiturates, BAER can persist in people and animals.19, 20, 21, 22 There have been no studies of the influence of lidocaine by any route of administration on BAER in horses or other species. Body temperature should be noted when using BAER as an aid to determine brain death because hypothermia can alter BAER by increasing interpeak intervals in people.23 The effect of temperature on the BAER in these horses was not investigated but was similar to results observed in the barbiturate study in horses.3 Absence and lack of recovery of any detectable brain electrical activity, based on EEG and BAER, supported the diagnosis of brain death in these horses.
Cardiovascular variables were the last to be lost. These included audible heart sounds, mean arterial pressure, and ECG activity. Heart sounds were undetectable from 3.7 to 10.2 minutes after the end of the infusion. At that time, brain death had already occurred. Despite undetectable heart sounds, palpable arterial pulse, and extremely low MAP, ECG activity was the last variable to be lost (after up to 21.3 minutes; Table 1). Electrocardiographic activity was lost up to 16.3 minutes after barbiturate infusion (Table 1).3 This apparently prolonged ECG activity represents electrical‐mechanical dissociation during which no effective cardiac contraction and output occur as evidenced by undetectable heart sounds and extremely low pulse pressure. Mean arterial pressure decreased to a nadir by 5 minutes postinfusion, indicating severe progressive loss of cardiac function. In the barbiturate study, 2 horses with the longest infusion times took the longest time to lose all ECG activity (16 minutes) postinfusion.3 Removing these 2 horses, ECG activity was lost up to 12 minutes after barbiturate infusion.3 In our lidocaine study, infusion time was set at ≤ 30 seconds to avoid time as a confounding factor in the sequence and timing of respiratory, cardiovascular, cerebral, and brainstem events.
Euthanasia means ending life in a painless and minimally distressful humane way.1 Horses in this study were anesthetized before CSF removal and infusion of lidocaine to minimize pain and distress while euthanasia was being performed. Although longer times to respiratory, cardiac and brainstem death were observed in this study compared to the barbiturate study,3 this method of euthanasia is already in practice in some parts of the world and was effective and resulted in relative rapid loss of consciousness (up to 3.8 minutes) followed by brainstem death and ultimately cardiac arrest. Based on our previous study, euthanasia by an overdose of barbiturate solution administered IV must be considered the first option in equine practice if available.3 However, results of our study showed that intrathecal administration of lidocaine hydrochloride under IV anesthesia could be used as an alternative method of euthanasia in situations in which other routine and approved methods are not available.
In conclusion, intrathecal lidocaine hydrochloride administration under IV anesthesia is an effective method of euthanasia, although it takes longer to induce respiratory, cardiovascular and brain death than does an IV overdose of barbiturates. We do not advocate its use instead of approved methods of euthanasia but rather provide objective information about a method already in use in other parts of the world where barbiturate solutions are not available or are cost prohibitive. Firearms are subject to strict regulations or are considered illegal, and captive bolt devices are expensive and often only approved for slaughter practices.1 It is not recommended to perform the method of euthanasia we have described without proper training because the procedure is technically challenging for the untrained practitioner. One major human health concern with this practice would be the risk of exposure to CSF taken from animals with an unknown disease, especially in countries where viral (eg, rabies) and other encephalitides are common and testing is not performed routinely. It is up to the professional judgment of the practicing veterinarian to consider the risks and benefits of this alternative method and to be cautious if it is elected. Furthermore, if this method is elected in the field, every effort to confirm death of the animal must be made.
Acknowledgments
The authors thank Dr Jairo Cardenas from Brazil for sharing his experience with this method of euthanasia, and Mrs. Cindy Davis for technical assistance.
Grant support: The study was supported by gifts from anonymous donors to the Comparative Neurology Research Group at UCD.
Conflict of Interest Declaration: Authors disclose no conflict of interest.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
The study was performed at the University of California at Davis.
Footnotes
Monger S. International Veterinary Consultants. Personal communication, 2013.
Cardenas J. Brazil: Personal communication, 2013.
Neurofax Wireless Input 1000A, Nihon Kohden America Inc., Foothill Ranch, CA
VikingQuest, Nicolet Biomedical Inc., Madison, WI
References
- 1. Leary S, Underwood W, Anthony R, et al. AVMA Guidelines for the euthanasia of animals: 2013 Edition. J Am Vet Med Assoc 2013:1–102. [Google Scholar]
- 2. Leary S, Underwood W, Raymond A, et al. AAEP Guidelines for Euthanasia. Lexington, KY: American Association of Equine Practitioners; 2011. [Google Scholar]
- 3. Aleman M, Williams DC, Guedes A, et al. Cerebral and brainstem electrophysiologic activity during euthanasia with pentobarbital sodium in horses. J Vet Intern Med 2015;29:663–672. doi:10.1111/jvim.12570:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Williams DC, Aleman M, Holliday TA, et al. Quantitative and qualitative characteristics of the electroencephalogram in normal horses following sedative administration. J Vet Intern Med 2012;26:645–653. [DOI] [PubMed] [Google Scholar]
- 5. Aleman M, Puchalski SM, Williams DC, et al. Brainstem auditory evoked responses in horses with temporohyoid osteoarthropathy. J Vet Intern Med 2008;22:1196–1202. [DOI] [PubMed] [Google Scholar]
- 6. Mayhew IG, de Lahunta A, Whitlock RH. Collection of cerebrospinal fluid from the horse. Cornell Vet 1975;65:500–511. [PubMed] [Google Scholar]
- 7. Pratt S, Hess P, Vasudevan A. A prospective randomized trial of lidocaine 30 mg versus 45 mg for epidural test dose for intrathecal injection in the obstretic population. Anesth Analg 2013;116:125–132. [DOI] [PubMed] [Google Scholar]
- 8. Zhao G, Ding X, Guo Y, et al. Intrathecal lidocaine neurotoxicity: Combination with bupivacaine and ropivacaine and effect of nerve growth factor. Life Sci 2014;112:10–21. [DOI] [PubMed] [Google Scholar]
- 9. Tauzin‐Fin P, Bernard O, Sesay M, et al. Benefits of intravenous lidocaine on post‐operative pain and acute rehabilitation after laparoscopic nephrectomy. J Anaesthesiol Clin Pharmacol 2014;30:366–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fikes LW, Lin HC, Thurmon JC. A preliminary comparison of lidocaine and xylazine as epidural analgesics in ponies. Vet Surg 1989;18:85–86. [DOI] [PubMed] [Google Scholar]
- 11. Borowicz KK, Banach M. Antiarrhythmic drugs and epilepsy. Pharmacol Rep 2014;66:545–551. [DOI] [PubMed] [Google Scholar]
- 12. Yingying S, Qinglin Y, Gang L, et al. Diagnosis of brain death: Confirmatory tests after clinical test. Chin Med J (Engl) 2014;127:1272–1277. [PubMed] [Google Scholar]
- 13. Monteiro LM, Bollen CW, van Huffelen AC, et al. Transcranial doppler ultrasonography to confirm brain death: A meta‐analysis. Intensive Care Med 2006;32:1937–1944. [DOI] [PubMed] [Google Scholar]
- 14. Sloan TB. Anesthetic effects on electrophysiologic recordings. J Clin Neurophysiol 1998;15:217–226. [DOI] [PubMed] [Google Scholar]
- 15. Spehlmann R. The normal BAEP In: Spehlmann R, ed. Evoked Potential Primer. Stoneham, MA: Butterworth Publishers; 1985:204–216. [Google Scholar]
- 16. Spehlmann R. Coma In: Spehlmann R, ed. Evoked Potential Primer. Stoneham, MA: Butterworth Publishers; 1985:217–235. [Google Scholar]
- 17. Aleman M, Holliday TA, Nieto JE, et al. Brainstem auditory evoked responses in an equine patient population. Part I: Adult horses. J Vet Intern Med 2014;28:1310–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Aleman M, Madigan JE, Williams DC, et al. Brainstem auditory evoked responses in an equine patient population. Part II: Foals. J Vet Intern Med 2014;28:1318–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stockard JJ, Stockard JE, Sharbrough FW. Nonpathological factors influencing brainstem auditory evoked potentials. Am J EEG Technol 1978;18:177. [Google Scholar]
- 20. Bobbin RP, May JG, Lemoine RL. Effects of pentobarbital and ketamine on brain stem auditory potentials: Latency and amplitude intensity functions after intraperitoneal administration. Arch Otolaryngol 1979;105:467–470. [DOI] [PubMed] [Google Scholar]
- 21. Sutton LN, Frewen T, Marsh R, et al. The effects of deep barbiturate coma on multimodality evoked potentials. J Neurosurg 1982;57:178–185. [DOI] [PubMed] [Google Scholar]
- 22. Marsh RR, Frewen TC, Sutton LN, et al. Resistane of the auditory brain stem response to high barbiturate levels. Otolaryngol Head Neck Surg 1984;92:685–688. [DOI] [PubMed] [Google Scholar]
- 23. Stockard JJ, Sharbrough FW, Tinker JA. Effects of hypothermia on the human brainstem auditory response. Ann Neurol 1978;3:368–370. [DOI] [PubMed] [Google Scholar]


