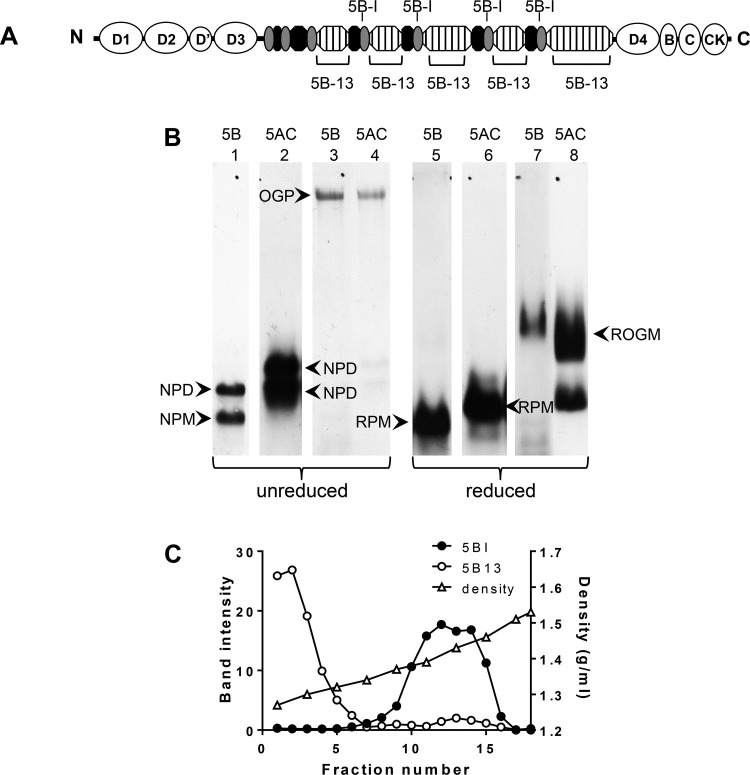
Fig. 1.
Identification of intracellular forms of MUC5B. A: cartoon of MUC5B NH2- and COOH-terminal domains (open ovals), repetitive glycosylated domains (hatched boxes), nonrepetitive glycosylated domains (solid ovals), and Cys domains (shaded ovals) highlighting the positions of the peptides used as immunogens for MAN-5BI and LUM5B-13. B: HBEC lysates, unreduced (lanes 1–4) or reduced by treatment with 10 mM DTT (lanes 5–8), were subjected to 0.7% (wt/vol) agarose gel electrophoresis and subsequently transferred to nitrocellulose. Western blots were probed with polyclonal antiserum LUM5B-13 (lanes 1 and 5), monoclonal antibody 2011 (lanes 2 and 6), polyclonal antiserum MAN-5BI (lanes 3 and 7) and polyclonal antiserum MAN-5ACI (lanes 4 and 8). The data presented were obtained from 2 gels and the details are given in experimental procedures. OGP, O-glycosylated polymeric mucin; ROGM, reduced O-glycosylated monomer; NPM, native polypeptide monomer; NPD, native polypeptide dimer; RPM, reduced polypeptide monomer. C: HBEC lysates were extracted with 6 M GdmCl and subjected to CsCl/4 M GdmCl density gradient centrifugation. After slot blotting onto nitrocellulose the non-O-glycosylated and O-glycosylated MUC5B distributions were analyzed by immunodetection with LUM5B-13 (○) and MAN-5BI (●), respectively. Fractions were also analyzed for density by weighing (△).