Figure 2.
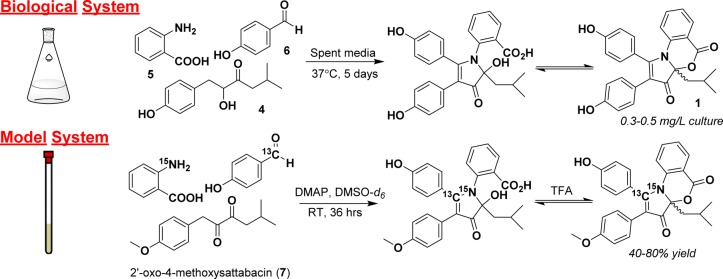
Initial studies of the formation of DPA in the fermentation flask were unsuccessful due to low reagent yields, interfering medium components, and complicated analytical sample preparation. To combat these issues, precursors were combined in deuterated organic solvent and observed constantly by NMR spectroscopy. The base 4-(dimethylamino)pyridine (DMAP) was used to simulate the basic environment of the fermentation flask (pH ∼9). The oxidation of the acyloin of 4-hydroxysattabacin to form a diketone is known to be the rate-limiting step in DPA formation, and thus, the diketone was prepared with Dess–Martin periodinane (DMP) beforehand to increase the efficiency of the model system.