Fig. 1.
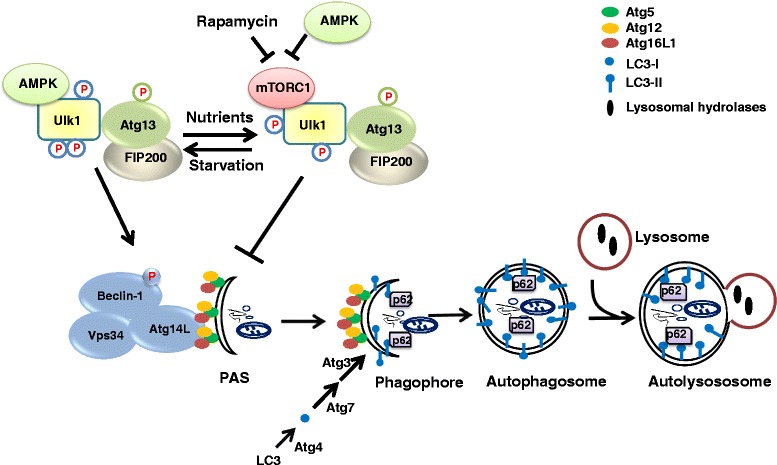
Macroautophagy is a major recycling pathway for long-lived proteins, protein aggregates and organelles. Autophagy induction is controlled by the AMPK/mTOR-signaling axis. In nutrient-rich conditions, AMPK activity is low and mTOR activity is high, thereby suppressing the autophagic pathway. Upon nutrient starvation and/or energy deprivation, AMPK activity is high, causing the inhibition of mTOR (directly and indirectly via the TSC1/2-Rheb GTPase pathway) and activation of the ULK1 complex involving a complex network of phosphorylations. mTOR inhibition and autophagy activation can also be achieved in nutrient-rich conditions using chemical mTOR inhibitors like rapamycin. Activation of the ULK1 complex contributes to the activation of the class III PI3K complex (Atg14L/Beclin 1 (Atg6)/Vps34 and the Vps34-regulatory protein Vps15 (not shown)) by phosphorylating Beclin 1, producing PtdIns(3)P from PtIns at the pre-autophagosomal structures (PAS) necessary for the formation of the phagophore. This structure then elongates into a double-membranous vesicle, the autophagosome, by recruitment of the Atg12/Atg5/Atg16 complex and the lipidation of LC3-I into phosphatidylethanolamine-conjugated LC3-II. LC3-II formation involves the production of LC3-I by Atg4-mediated proteolytic cleavage of LC3 and the action of E1-like ligases (Atg7), E2-like ligases (Atg3) and E3-like ligase complexes (Atg12/Atg5/Atg16L1). Furthermore, ubiquitin-binding proteins like p62 can bind LC3, thereby linking ubiquitinated proteins to LC3-II and targeting them for autophagic degradation. The Atg12/Atg5/Atg16 complex present in the outer membrane dissociates from mature autophagosomes, which then fuse with the lysosomes, degrading the inner membrane and the cargo present in the autophagosomes via luminal hydrolysases and subsequently releasing the digested material via permeases back into the cytosol. The fusion of autophagosomes with lysosomes can also involve endosomes, leading to intermediate amphisome formation
