Biological correlates of internalizing symptoms have long been sought, in efforts to advance our understanding of their underlying brain mechanisms and links to illness progression (see Foland-Ross, Hardin, & Gotlib, 2013 for recent reviews). Identifying these correlates in early childhood is essential, as early internalizing problems increase risk for subsequent psychopathology in adolescence and adulthood (Mesman & Koot, 2001). Hypothalamic Pituitary Adrenal (HPA) Axis functioning, specifically the Cortisol Awakening Response, has been proposed as a mechanism of risk for childhood and adolescent internalizing psychopathology (CAR; (Adam et al., 2010; Dietrich et al., 2013). However, no study has explored the link between CAR and internalizing problems among children younger than 8 years of age.
CAR reflects the acute cortisol increase in the first 30 to 60 minutes after waking and likely represents an adaptive component of the transition to wakefulness with heightened CAR perhaps preparing and facilitating daily engagement with an individual’s social environment (Fries, Dettenborn, & Kirschbaum, 2009). Speculations on mechanistic pathways for atypical CAR in depressed youth suggest dysregulated HPA-axis functioning such as impairment to negative feedback regulation (Adam, 2010) and/or heightened excitatory signaling such as higher levels of exposed (Doane and Adam, 2010) or anticipatory stress (Adam, 2006; Greaves-Lord et al., 2007; (Griefahn & Robens, 2011)(Rohleder et al., 2006). CAR emerges early in life (Gribbin, Watamura, Cairns, Harsh, & Lebourgeois, 2011) and has demonstrated stability from 1 to 7 years (Bäumler, Kirschbaum, Kliegel, Alexander, & Stalder, 2013). To date, evidence of its connection to internalizing psychopathology in late childhood and adolescence has been somewhat mixed. For example, CAR was positively associated with internalizing symptoms among a clinically referred sample of 10–12 year old children (Dietrich et al., 2013), but negatively associated with cognitive symptoms of anxiety among boys in a community sample of 10–12 year-olds in the same study (Dietrich et al., 2013). With older adolescents, higher CAR increased risk for developing depression and/or anxiety one year later (Adam et al., 2010, 2014), yet other studies found correlations with only specific current depression symptom clusters (Bosch et al., 2009; Shibuya et al., 2014), or no correlation with depressive/anxiety symptoms (Dietrich et al., 2013; Hartman, Hermanns, de Jong, & Ormel, 2013a).
These studies vary on the internalizing outcome assessed and inclusion of comorbid diagnoses (with or without controlling for externalizing symptoms) (see Adams et al., 2010; (Dietrich et al., 2013; Greaves-Lord et al., 2007; Hartman, Hermanns, de Jong, & Ormel, 2013b; Kallen et al., 2008; Young, Sweeting, & West, 2012). Prior work has noted the likely complex effects of co-occurring symptoms on cortisol patterns (McBurnett et al., 1991). Among children, studies have primarily focused on externalizing disorders and HPA-axis reactivity while controlling for the presence of co-occurring internalizing symptoms ((Marsman et al., 2008; McBurnett et al., 1991; van Goozen et al., 1998), so little is known about how externalizing symptoms may affect any internalizing-HPA axis associations. On their own, externalizing symptoms have most often demonstrated links with blunted cortisol (Platje et al., 2013; Shirtcliff et al., 2009; van Goozen et al., 1998), however these results have not been examined extensively by childhood age, and have focused mainly on predominantly “pure”, persistent, chronic aggression. Given the high rates of co-occurrence of internalizing and externalizing symptoms in childhood it is especially important to assess and include both types in analyses with children (Costello, Mustillo, Erkanli, Keeler, & Angold, 2003).
Additionally, age may influence the relationship between CAR and internalizing symptomatology. Areas of the brain implicated in both the cortisol response and internalizing symptoms (e.g., the hippocampus) experience maturation during middle childhood (adrenarche) (see Herbert, 2012), which could lead to changes in the CAR-internalizing association. Also, cortisol reactivity to psychosocial stressors, an index of HPA functioning studied more extensively with children (Hankin, Badanes, Abela, & Watamura, 2010; Luby et al., 2003; Suzuki, Belden, Spitznagel, Dietrich, & Luby, 2013), has shown differential associations with internalizing/dysphoric symptoms at specific developmental stages, with symptoms related to hypo-reactivity in preschoolers, and unrelated to reactivity in middle childhood. Conflicting results may be due to the maturation of the HPA-axis, increasing cognitive and socio-emotional capacities (Del Giudice, Ellis, & Shirtcliff, 2011), and/or because different stressors had to be used to be developmentally appropriate (Hankin et al., 2010). CAR provides a useful index to explore age-related effects in the HPA-internalizing link among children, as it may be less influenced by changes in self-regulation or coping and does not require paradigm changes for appropriate use with young and older children.
Here, we examine the link between CAR and internalizing symptoms in at-risk children 22 to 96 months old while parsing out the influence of externalizing symptoms and child age. We also explore whether child age moderates the relationship between internalizing symptoms and CAR, as suggested by studies using other indices of HPA-axis functioning. Because previous studies with this population are lacking, and studies with older children report inconsistent findings, our aims are exploratory and we did not hypothesize specific directionality for these associations.
Method
Participants
Fifty-two children (53.8% female) participated, aged 22 to 96 months (Mmo = 58.60, SDmo = 20.66, eight aged 22–35 months, 11 aged 36–47 months, six aged 48–59 months, nine aged 60–71 months, 11 aged 72–83 months, 7 aged 84–96 months). Of these, 11.5% had family incomes below $15,000 annually, and 42.3% made over $100,000 per year. The sample was 67% Caucasian, 19% bi-racial, and 12% African American.
Participants were recruited for this cross-sectional study from two longitudinal NIMH funded studies of psychiatrically at-risk mothers and their children. Those studies oversampled for either perinatal depression (study P, project title and PI deleted for blind review) or exposure to maternal childhood maltreatment (study M, project title and PI deleted for blind review). Of participating mothers, 21% had a diagnosis of Posttraumatic Stress Disorder (PTSD) diagnosis, and 15% met criteria for mild to moderate depression. Of the 52 children, 13 (25%) scored in the clinical or borderline clinical range for externalizing (n=6), internalizing symptoms (n=3), or both types (n=4) on the CBCL (T>65).
Procedures
Mothers were mailed child symptom, maternal symptom and demographic questionnaires, Salivette tubes (Sarstedt), collection instructions, and a ‘saliva collection log’ in which times of collections and other methodological variables known to influence child CAR were recorded: collection problem (e.g. child ate, drank, or was sick); atypical routine (i.e., holiday, babysitter drove children to school); medication use; wake time, and minutes between sample collections. Saliva was collected “upon waking” (CAR1), and “45 minutes after waking” (CAR2) across two consecutive days. Saliva samples were centrifuged for 10 minutes at 7000 rpm, and stored at −80* C until assayed. Cortisol was assayed in duplicate using ELISA kits with a sensitivity of .003 ug/dL. The intra-assay and inter-assay coefficients of variability were 10% and 3%, respectively.
Measures
Child symptoms
The Child Behavior Checklist (CBCL, Achenbach & Rescorla, 2000, 2001) is a parent-completed questionnaire widely used to assess internalizing and externalizing problems. It is normed by age and gender (T scores), with slightly different versions needed to capture internalizing and externalizing behaviors at different ages (one for ages 1.5 to 5 and another for ages 6 to 18). Chronbach’s alphas for internalizing and externalizing scales were .87 and .90 for the CBCL 1.5–5, and .80 and .75 for the CBCL 6–18, respectively.
Maternal Symptoms
Maternal depressive symptoms were assessed with the Beck Depression Inventory-II (Beck, Steer, & Brown, 1996). Post-Traumatic Stress Disorder symptoms were assessed using the National Women's Study PTSD Module (Resnick, Kilpatrick, Dansky, Saunders, & Best, 1993). Chronbach’s alphas were .84 and .88.
Statistical Analyses
Data analysis
We constructed multiple models to examine the associations between CAR magnitude and possible methodological and environmental factors and problem behaviors. For each analysis, we measured CAR magnitude (i.e., change) using a mixed linear regression model framework (SPSS 21) predicting to CAR change (CAR2-CAR1) from the predictors of interest while controlling for CAR1. Cortisol data was transformed, based on the Box-Cox Transformation Lambda =.26, which has been found to be optimal for this type of salivary cortisol data (Miller & Plessow, 2013). Effect sizes for significant results were calculated using a modification of Cohen’s f2 for mixed effects models (Selya, Rose, Dierker, Hedeker, & Mermelstein, 2012).
Results
Descriptive Statistics
Descriptive statistics for cortisol levels at awakening and 45-minutes post-wake as well as for methodological variables (collection problem, atypical day, medication use, and wake time) are reported in Table 1. Of the 52 children, eight (15%) provided saliva samples on only one of the days. Thus, of the total 96 days examined, 37 days (39%) exhibited a rise (10% increase) in morning cortisol levels after awakening.
Table 1.
Descriptive Statistics: Cortisol Levels and Methodological Covariates
| Methodological Covariates | Day 1 (n=49) n or M (% or SD) |
Day 2 (n=47) n or M (% or SD) |
|---|---|---|
| Atypical Routine | 10 (20%) | 6 (12.7%) |
| Collection Problem | 10 (20%) | 10 (21.3%) |
| Medication | 4 (8.2%) | 5 (10.6%) |
| Minutes Between Samples (10m–198m) | 44.73 (11.59) | 45.87 (11.72) |
| Wake Time (5:55am–12:00pm) | 8:02am (1:17) | 8:05am (1:06) |
| Cortisol 1 Value (Range: .02–.89) | .26 (.17) | .24 (.13) |
| Cortisol 2 Value (Range: .02–.55) | .24 (.11) | .24 (.13) |
Note: Covariate details were as follows: Atypical routines (atypical vs typical) commonly included it being a holiday, or small changes in morning routine such as the babysitter driving the children to school instead of the mother. Collection problems (yes vs no) included child eating, drinking, or being sick prior to samples. Medication (yes vs no) describes whether the child took medications prior to samples. Weekend collection meant cortisol was collected on weekend morning vs not (weekday morning).
Analysis of Covariates
Prior to hypothesis testing we examined whether CAR magnitude was predicted by methodological and environmental (maternal psychopathology/income level) covariates in separate models. Collection day being a weekend (b = 0.038, t(76.63)=2.00, p=.049) significantly predicted CAR magnitude only in an adjusted model with typical day included (see Table 2). Both variables were therefore included as covariates in subsequent models. No other methodological or environmental covariates had significant main effects in adjusted or unadjusted models (see Table 3).
Table 2.
Mixed effects model of Cortisol Awakening Response Magnitude as predicted by Methodological Covariates (N=52)
| Covariates | Reference | Beta [Conf. Intervals] | t | p |
|---|---|---|---|---|
| Methodological Covariate Model | ||||
| Cortisol Waking Sample | −.772 [−.90, −.64] | −11.66 | .000** | |
| Minutes Between Samples | .000 [−.00, .00] | .92 | .360 | |
| Wake Time | −.000 [−.00, −.00] | − .63 | .528 | |
| Collection Problem | No Problems | −.045 [−.10, .00] | −1.90 | .062 |
| Typical Day | Typical | .035 [−.01, .08] | 1.48 | .142 |
| Medications | No Meds | −.031 [−.10, .04] | − .88 | .385 |
| Day of Week Collection | Weekend | .039 [ .00, .08] | 2.00 | .049* |
| Environmental Covariate Model | ||||
| Cortisol Waking Sample | −.752 [−.89, −.62] | −11.18 | .000** | |
| Typical Day | Typical | .042 [−.01, .09] | 1.75 | .084 |
| Day of Week Collection | Weekend | .023 [−.02, .06] | 1.12 | .265 |
| Maternal Depressive Symptoms | −.002 [−.01, .00] | −.66 | .513 | |
| Maternal PTSD Symptoms | .003 [−.00, .01] | 1.03 | .310 | |
| Family Income Level | −.002 [−.01, .00] | −.95 | .345 | |
Note: Family income was measured annually on a scale of 0–21 with $5,000 range increments from “$0–5,000” to “above $100,000”.
Table 3.
Mixed effects model of Cortisol Awakening Response Magnitude as predicted by Child Age, Internalizing and Externalizing Symptoms (N=52)
| Covariates | Reference | Beta [Confidence Intervals] | t | p |
|---|---|---|---|---|
| Cortisol Waking Sample | −.782 [−.91, −.66] | −12.32 | .000** | |
| Typical Day | Typical | .047 [−.00, .09] | 1.94 | .056 |
| Collection Day of Week | Weekend | .028 [−.01, .07] | 1.50 | .139 |
| Child Age (Months) | −.005 [−.01, −.00] | −2.34 | .024* | |
| Child Externalizing Symptoms | .005 [−.00, .01] | 2.87 | .006** | |
| Child Age * Internalizing | .011 [−.02,−.00] | −3.37 | .001** | |
| Child Age * Internalizing | .0001 [.00, .00] | 2.55 | 014* | |
| Symptoms Interaction |
Note.
p<.05,
p<.01
Main Effects of Age and Co-occurring Externalizing Symptoms
We first examined a model of internalizing symptoms and child age predicting CAR magnitude while controlling for typical day, collection problems and day of the week collection. In this model, neither internalizing symptoms, b = −0.0004, t(44.59) = −.368, p = 0.714 nor age b = 0.0003, t(42.52) = .596, p = 0.554 significantly predicted CAR magnitude.. However, when externalizing symptoms were added to the model, higher internalizing symptoms were associated with lower CAR magnitude, b = −0.004, t(44.42) = −2.216, p = 0.032, f2 = .063 and higher externalizing symptoms were associated with greater CAR magnitude b = 0.005, t(45.54) = 2.531, p = 0.015, f2 = .064. Post hoc analyses of a separate model with externalizing symptoms, age and covariates showed externalizing symptoms alone were also not associated with CAR, b= 0.002, t(47.70) = 1.30, p = 0.200.
Age-by-Symptoms Interactions
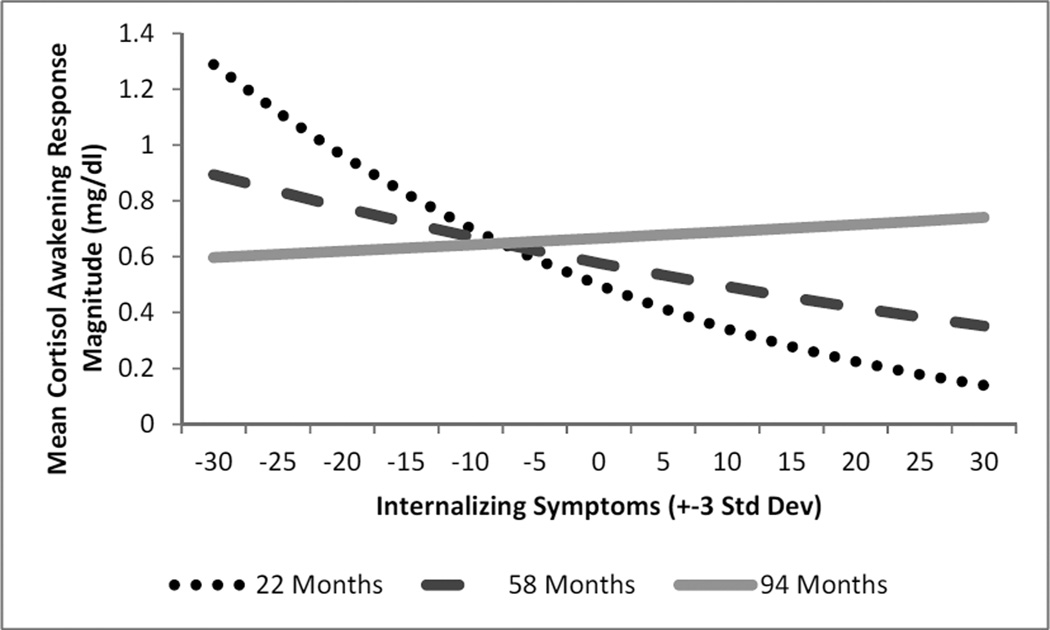
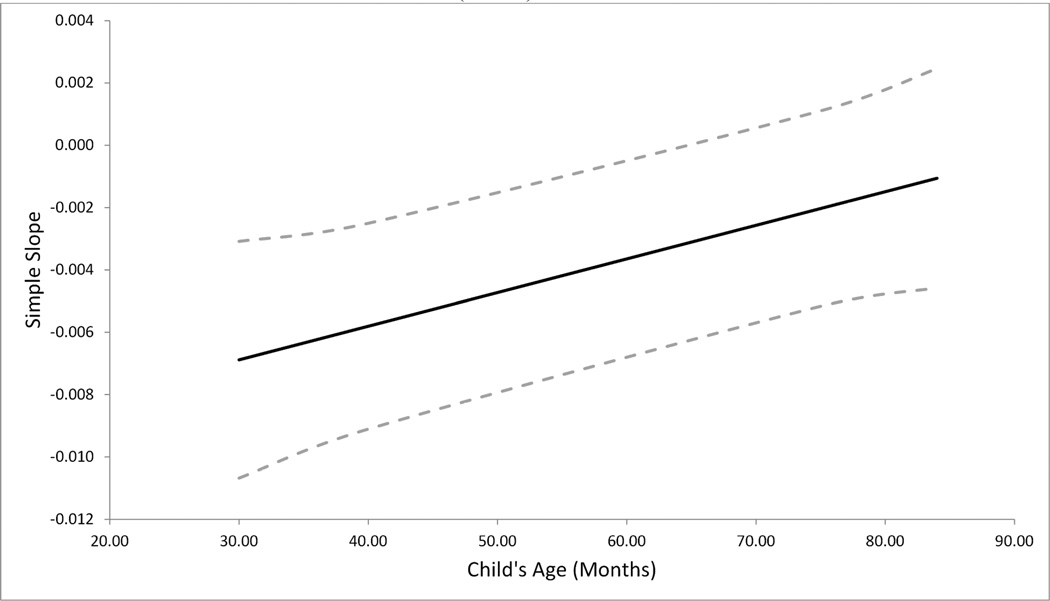
Adding an age by internalizing symptoms interaction revealed a significant effect, Age × Internalizing b = 0.00012, t(42.80) = 2.55, p = 0.014, f2 = .069 (see Table 3). While internalizing symptoms were associated with lower CAR magnitude in early childhood from age 22 months until 66 months, this relationship disappeared with age (see Figure 1). Calculation of regions of significance of simple slopes indicate that the impact of internalizing symptoms on CAR (slope) became non-significant starting at 67 months (b = −0.0028, t(80) = −1.97, p = 0.052) (see Figure 2). Additional analyses showed there was no difference of internalizing symptoms between children younger and older than 66 months t(89.83)= −0.63, p=.531. Exploratory analyses revealed that when tested in a separate model, there was no significant interaction of age by externalizing symptoms (b = .00012, t(49.12) = 1.97, p = 0.061) on CAR magnitude.
Figure 1.
Estimated Curves for Modeling of Internalizing Symptoms and Cortisol Awakening Response Magnitude Moderated by Child Age controlling for Externalizing Symptoms (N=52)
Note: Separate lines depict model estimation at three different ages, 22 (dotted line-youngest age in our sample), 58 (dashed line-mean age of our sample) and 94 months old (solid lineoldest age in our sample).
Figure 2.
Simple slope and 95% confidence bands of the impact of internalizing symptoms on Cortisol Awakening Response across early childhood. The effect becomes non-significant when confidence bands reach 0 after 66 months (N=52)
Note: The solid line represent the simple slope and the dashed lines represent its 95% confidence bands.
Discussion
We examined the impact of internalizing symptoms on children’s Cortisol Awakening Response among at-risk 22- to 96-months-olds while controlling for externalizing symptoms and child age as covariates. We found an inverse association between internalizing symptoms and CAR magnitude, which became non-significant starting at 67 month and remained non-significant thereafter. This is the first study to examine the relationship between internalizing symptoms and CAR in early childhood. Our finding that blunted CAR is linked to higher internalizing symptoms early in life is in contrast to similar studies of middle childhood and adolescents (Adam et al., 2010; Dietrich et al., 2013; Greaves-Lord et al., 2007; Ruttle et al., 2011). However, a similar pattern of internalizing symptoms linked to low cortisol in young children and unrelated to cortisol in middle childhood has been reported in the stress reactivity literature (Hankin et al., 2010). Past researchers finding blunted cortisol in prepubertal children at risk for depression have speculated 1) that at-risk children with hyporeactivity have less adaptive stress response systems, which may result in less energy to cope with stressors sufficiently (Hankin et al., 2010) or 2) that blunted cortisol may be caused by a down-regulation of the adrenergic system due to over-activation of the system from either chronic stress, and/or pervasive depressed mood (Hankin et al., 2010; Suzuki et al., 2013). It is also possible that both CAR and internalizing symptoms may be influenced by developmental changes in sleep-wake patterns, since sleep and CAR (Gribbin et al., 2011; Pesonen et al., 2012), and sleep and internalizing problems (Gregory, Rijsdijk, Dahl, McGuffin, & Eley, 2006) are highly related. For instance, in eight year old boys, sleep disturbances (sleep-wake transition problems, excessive waking sleepiness, and excessive bedtime sweating), are associated with lower CAR (Pesonen et al., 2012). Twenty-five percent of children have sleep disturbances like these, and only about half persist across development (Pesonen et al., 2012), potentially mediating the CAR-internalizing association early in life.
The internalizing symptoms-CAR link was only seen when controlling for externalizing symptoms, which predicted greater CAR magnitude. Thus, modeling internalizing symptoms alone may allow for uncontrolled (positive) effects of externalizing symptoms to be present which would nullify direct (negative) effects of internalizing problems, coined a suppression effect (MacKinnon, Krull, & Lockwood, 2000). Thus, it seems critical for future studies on internalizing symptoms and cortisol to control for externalizing symptoms in childhood. The link between externalizing symptoms and HPA-axis functioning is quite complex (Alink et al., 2008). While some work, primarily conducted among adolescent, is linking low CAR to callousness (Shirtcliff et al., 2009) and persistent aggressiveness (Platje et al., 2013), other research suggests greater HPA-axis reactivity associated with certain types of externalizing behaviors, specifically, reactive aggression (Lopez-Duran, Olson, Hajal, Felt, & Vazquez, 2009) and non-chronic externalizing symptoms (Marsman et al., 2008), which are more characteristic of young children (Mesman et al., 2009). This age-effect may also explain the present finding of higher CAR related to externalizing symptoms. Fries and colleagues (2009), suggest that an increased CAR facilitates engagement with social environment for that day, thus it may be that young children with non-chronic externalizing symptoms are over-responding to their social environment.
This study has several limitations. The sample was over-selected for high levels of maternal psychopathology, and children had relatively low rates of psychopathology so results may not generalize to a broader population. Objective measures of waking or saliva collection timing were not utilized and late collection can result in inaccurate CAR assessment (Griefahn & Robens, 2011), reflecting parental compliance instead of true HPA-axis activity where lower compliance would result in blunted CAR (Wright & Steptoe, 2005). However, high externalizing problems were associated with increased CAR, suggesting heightened compliance in our sample, which is not what one would expect. Additionally, two developmentally appropriate but distinct CBCL versions were used to assess child symptoms and thus it is possible that differences in items included in the internalizing scale between the two versions impacted our age-related findings. It is also important to note that the CBCL internalizing scale utilized includes symptoms of both anxiety and depression, which may have distinct effects on the HPA-axis, however attempts to separate them within the CBCL have been problematic (Dingle et al., 2011; Ebesutani et al., 2010) and thus were not examined here. Sex differences are also of interest; however, due to limitations of the sample size, we could not test this interaction. Additionally, other potentially moderating variables were omitted from our methods such as chronicity and onset of symptoms as well as antecedent stressful experiences, which would benefit from future research. Future research should also test the observed age-related differences using longitudinal samples. Finally, the effect size of our findings were relatively small (Cohen’s f2 = .06–.07). Given these limitations, results must be considered tentative and replication is needed. Our data demonstrate the potential value of examining CAR and internalizing symptoms in early childhood while controlling for externalizing symptoms, but further work is clearly needed to address the interaction of this link across development.
Contributor Information
Ellen W McGinnis, University of Michigan – Psychology, 530 Church St, Ann Arbor, Michigan 48109, United States.
Nestor Lopez-Duran, University of Michigan – Psychology, 530 Church St, Ann Arbor, Michigan 48109, United States.
Cecilia Martinez-Torteya, Depaul University – Psychology, Chicago, Illinois, United States.
James L Abelson, University of Michigan – Psychiatry, Ann Arbor, United States.
Maria Muzik, University of Michigan – Psychiatry, Ann Arbor, United States.
References
- Achenbach TM, Rescorla LA. Manual for the ASEBA Preschool FormPreschool Forms & Profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, and Families; 2000. [Google Scholar]
- Achenbach TM, Rescorla LA. ASEBA School Age Forms and Profiles. Burlington, Vt: ASEBA; 2001. Retrieved from http://www.childbehaviorchecklist.com/ordering/ASEBA%20Reliability%20and%20Validity-School%20Age.pdf. [Google Scholar]
- Adam EK, Doane LD, Zinbarg RE, Mineka S, Craske MG, Griffith JW. Prospective prediction of major depressive disorder from cortisol awakening responses in adolescence. Psychoneuroendocrinology. 2010;35(6):921–931. doi: 10.1016/j.psyneuen.2009.12.007. http://doi.org/10.1016/j.psyneuen.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam EK, Vrshek-Schallhorn S, Kendall AD, Mineka S, Zinbarg RE, Craske MG. Prospective associations between the cortisol awakening response and first onsets of anxiety disorders over a six-year follow-up--2013 Curt Richter Award Winner. Psychoneuroendocrinology. 2014;44:47–59. doi: 10.1016/j.psyneuen.2014.02.014. http://doi.org/10.1016/j.psyneuen.2014.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alink LRA, van Ijzendoorn MH, Bakermans-Kranenburg MJ, Mesman J, Juffer F, Koot HM. Cortisol and externalizing behavior in children and adolescents: mixed meta-analytic evidence for the inverse relation of basal cortisol and cortisol reactivity with externalizing behavior. Developmental Psychobiology. 2008;50(5):427–450. doi: 10.1002/dev.20300. http://doi.org/10.1002/dev.20300. [DOI] [PubMed] [Google Scholar]
- Bäumler D, Kirschbaum C, Kliegel M, Alexander N, Stalder T. The cortisol awakening response in toddlers and young children. Psychoneuroendocrinology. 2013 doi: 10.1016/j.psyneuen.2013.05.008. http://doi.org/10.1016/j.psyneuen.2013.05.008. [DOI] [PubMed] [Google Scholar]
- Beck A, Steer R, Brown G. Manual for the Beck Depression Inventory–II. San Antonio, TX: 1996. [Google Scholar]
- Bosch NM, Riese H, Dietrich A, Ormel J, Verhulst FC, Oldehinkel AJ. Preadolescents’ somatic and cognitive-affective depressive symptoms are differentially related to cardiac autonomic function and cortisol: the TRAILS study. Psychosomatic Medicine. 2009;71(9):944–950. doi: 10.1097/PSY.0b013e3181bc756b. http://doi.org/10.1097/PSY.0b013e3181bc756b. [DOI] [PubMed] [Google Scholar]
- Clow A, Thorn L, Evans P, Hucklebridge F. The Awakening Cortisol Response: Methodological Issues and Significance. Stress: The International Journal on the Biology of Stress. 2004;7(1):29–37. doi: 10.1080/10253890410001667205. [DOI] [PubMed] [Google Scholar]
- Costello EJ, Mustillo S, Erkanli A, Keeler G, Angold A. Prevalence and Development of Psychiatric Disorders in Childhood and Adolescence. Archives of General Psychiatry. 2003;60(8):837–844. doi: 10.1001/archpsyc.60.8.837. http://doi.org/10.1001/archpsyc.60.8.837. [DOI] [PubMed] [Google Scholar]
- Del Giudice M, Ellis BJ, Shirtcliff EA. The Adaptive Calibration Model of stress responsivity. Neuroscience and Biobehavioral Reviews. 2011;35(7):1562–1592. doi: 10.1016/j.neubiorev.2010.11.007. http://doi.org/10.1016/j.neubiorev.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich A, Ormel J, Buitelaar JK, Verhulst FC, Hoekstra PJ, Hartman CA. Cortisol in the morning and dimensions of anxiety, depression, and aggression in children from a general population and clinic-referred cohort: An integrated analysis. The TRAILS study. Psychoneuroendocrinology. 2013;38(8):1281–1298. doi: 10.1016/j.psyneuen.2012.11.013. http://doi.org/10.1016/j.psyneuen.2012.11.013. [DOI] [PubMed] [Google Scholar]
- Dingle K, Clavarino A, Williams GM, Bor W, Najman JM, Alati R. Predicting depressive and anxiety disorders with the YASR internalising scales (empirical and DSM-oriented) Social Psychiatry and Psychiatric Epidemiology. 2011;46(12):1313–1324. doi: 10.1007/s00127-010-0303-2. http://doi.org/10.1007/s00127-010-0303-2. [DOI] [PubMed] [Google Scholar]
- Dockray S, Bhattacharyya MR, Molloy GJ, Steptoe A. The cortisol awakening response in relation to objective and subjective measures of waking in the morning. Psychoneuroendocrinology. 2008;33(1):77–82. doi: 10.1016/j.psyneuen.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Ebesutani C, Bernstein A, Nakamura BJ, Chorpita BF, Higa-McMillan CK, Weisz JR. Concurrent Validity of the Child Behavior Checklist DSM-Oriented Scales: Correspondence with DSM Diagnoses and Comparison to Syndrome Scales. Journal of Psychopathology and Behavioral Assessment. 2010;32(3):373–384. doi: 10.1007/s10862-009-9174-9. http://doi.org/10.1007/s10862-009-9174-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A. Neurobiology of anxiety: from neural circuits to novel solutions? Depression and Anxiety. 2012;29(5):355–358. doi: 10.1002/da.21957. http://doi.org/10.1002/da.21957. [DOI] [PubMed] [Google Scholar]
- Foland-Ross LC, Hardin MG, Gotlib IH. Neurobiological markers of familial risk for depression. Current Topics in Behavioral Neurosciences. 2013;14:181–206. doi: 10.1007/7854_2012_213. http://doi.org/10.1007/7854_2012_213. [DOI] [PubMed] [Google Scholar]
- Forbes EE, Bertocci MA, Gregory AM, Ryan ND, Axelson DA, Birmaher B, Dahl RE. Objective sleep in pediatric anxiety disorders and major depressive disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2008;47(2):148–155. doi: 10.1097/chi.0b013e31815cd9bc. http://doi.org/10.1097/chi.0b013e31815cd9bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries E, Dettenborn L, Kirschbaum C. The cortisol awakening response (CAR): facts and future directions. International Journal of Psychophysiology. 2009;72(1):67–73. doi: 10.1016/j.ijpsycho.2008.03.014. [DOI] [PubMed] [Google Scholar]
- Gilliom M, Shaw DS. Codevelopment of externalizing and internalizing problems in early childhood. Development and Psychopathology. 2004;16(2):313–333. doi: 10.1017/s0954579404044530. http://doi.org/10.1017/S0954579404044530. [DOI] [PubMed] [Google Scholar]
- Greaves-Lord K, Ferdinand RF, Oldehinkel AJ, Sondeijker FEPL, Ormel J, Verhulst FC. Higher cortisol awakening response in young adolescents with persistent anxiety problems. Acta Psychiatrica Scandinavica. 2007;116(2):137–144. doi: 10.1111/j.1600-0447.2007.01001.x. http://doi.org/10.1111/j.1600-0447.2007.01001.x. [DOI] [PubMed] [Google Scholar]
- Gregory AM, Caspi A, Eley TC, Moffitt TE, O’Connor TG, Poulton R. Prospective Longitudinal Associations Between Persistent Sleep Problems in Childhood and Anxiety and Depression Disorders in Adulthood. Journal of Abnormal Child Psychology: An Official Publication of the International Society for Research in Child and Adolescent Psychopathology. 2005;33(2):157–163. doi: 10.1007/s10802-005-1824-0. http://doi.org/10.1007/s10802-005-1824-0. [DOI] [PubMed] [Google Scholar]
- Gregory AM, Rijsdijk FV, Dahl RE, McGuffin P, Eley TC. Associations between sleep problems, anxiety, and depression in twins at 8 years of age. Pediatrics. 2006;118(3):1124–1132. doi: 10.1542/peds.2005-3118. http://doi.org/10.1542/peds.2005-3118. [DOI] [PubMed] [Google Scholar]
- Gribbin CE, Watamura SE, Cairns A, Harsh JR, Lebourgeois MK. The cortisol awakening response (CAR) in 2- to 4-year-old children: Effects of acute nighttime sleep restriction, wake time, and daytime napping. Developmental Psychobiology. 2011 doi: 10.1002/dev.20599. http://doi.org/10.1002/dev.20599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griefahn B, Robens S. Cortisol awakening response - Are sampling delays of 15 minutes acceptable? International Journal of Psychophysiology. 2011;82(2):202–205. doi: 10.1016/j.ijpsycho.2011.08.005. http://doi.org/10.1016/j.ijpsycho.2011.08.005. [DOI] [PubMed] [Google Scholar]
- Hankin BL, Badanes LS, Abela JRZ, Watamura SE. Hypothalamic-Pituitary-Adrenal Axis Dysregulation in Dysphoric Children and Adolescents: Cortisol Reactivity to Psychosocial Stress from Preschool Through Middle Adolescence. Biological Psychiatry. 2010;68(5):484–490. doi: 10.1016/j.biopsych.2010.04.004. http://doi.org/10.1016/j.biopsych.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman CA, Hermanns VW, de Jong PJ, Ormel J. Self- or parent report of (co-occurring) internalizing and externalizing problems, and basal or reactivity measures of HPA-axis functioning: A systematic evaluation of the internalizing-hyperresponsivity versus externalizing-hyporesponsivity HPA-axis hypothesis. Biological Psychology. 2013a;94(1):175–184. doi: 10.1016/j.biopsycho.2013.05.009. http://doi.org/10.1016/j.biopsycho.2013.05.009. [DOI] [PubMed] [Google Scholar]
- Hartman CA, Hermanns VW, de Jong PJ, Ormel J. Self- or parent report of (co-occurring) internalizing and externalizing problems, and basal or reactivity measures of HPA-axis functioning: A systematic evaluation of the internalizing-hyperresponsivity versus externalizing-hyporesponsivity HPA-axis hypothesis. Biological Psychology. 2013b;94(1):175–184. doi: 10.1016/j.biopsycho.2013.05.009. http://doi.org/10.1016/j.biopsycho.2013.05.009. [DOI] [PubMed] [Google Scholar]
- Hicks BM, Patrick CJ. Psychopathy and negative emotionality: analyses of suppressor effects reveal distinct relations with emotional distress, fearfulness, and anger-hostility. Journal of Abnormal Psychology. 2006;115(2):276–287. doi: 10.1037/0021-843X.115.2.276. http://doi.org/10.1037/0021-843X.115.2.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallen VL, Tulen JHM, Utens EMWJ, Treffers PDA, De Jong FH, Ferdinand RF. Associations between HPA axis functioning and level of anxiety in children and adolescents with an anxiety disorder. Depression and Anxiety. 2008;25(2):131–141. doi: 10.1002/da.20287. http://doi.org/10.1002/da.20287. [DOI] [PubMed] [Google Scholar]
- Lopez-Duran NL, Olson SL, Hajal NJ, Felt BT, Vazquez DM. Hypothalamic pituitary adrenal axis functioning in reactive and proactive aggression in children. Journal of Abnormal Child Psychology. 2009;37(2):169–182. doi: 10.1007/s10802-008-9263-3. http://doi.org/10.1007/s10802-008-9263-3. [DOI] [PubMed] [Google Scholar]
- Luby JL, Heffelfinger A, Mrakotsky C, Brown K, Hessler M, Spitznagel E. Alterations in stress cortisol reactivity in depressed preschoolers relative to psychiatric and no-disorder comparison groups. Archives of General Psychiatry. 2003;60(12):1248–1255. doi: 10.1001/archpsyc.60.12.1248. http://doi.org/10.1001/archpsyc.60.12.1248. [DOI] [PubMed] [Google Scholar]
- MacKinnon DP, Krull JL, Lockwood CM. Equivalence of the mediation, confounding and suppression effect. Prevention Science: The Official Journal of the Society for Prevention Research. 2000;1(4):173–181. doi: 10.1023/a:1026595011371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsman R, Swinkels SHN, Rosmalen JGM, Oldehinkel AJ, Ormel J, Buitelaar JK. HPA-axis activity and externalizing behavior problems in early adolescents from the general population: the role of comorbidity and gender The TRAILS study. Psychoneuroendocrinology. 2008;33(6):789–798. doi: 10.1016/j.psyneuen.2008.03.005. http://doi.org/10.1016/j.psyneuen.2008.03.005. [DOI] [PubMed] [Google Scholar]
- McBurnett K, Lahey BB, Frick PJ, Risch C, Loeber R, Hart EL, Hanson KS. Anxiety, inhibition, and conduct disorder in children: II. Relation to salivary cortisol. Journal of the American Academy of Child and Adolescent Psychiatry. 1991;30(2):192–196. doi: 10.1097/00004583-199103000-00005. http://doi.org/10.1097/00004583-199103000-00005. [DOI] [PubMed] [Google Scholar]
- Mesman J, Koot HM. Early preschool predictors of preadolescent internalizing and externalizing DSM-IV diagnoses. Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40(9):1029–1036. doi: 10.1097/00004583-200109000-00011. http://doi.org/10.1097/00004583-200109000-00011. [DOI] [PubMed] [Google Scholar]
- Mesman J, Stoel R, Bakermans-Kranenburg MJ, van IJzendoorn MH, Juffer F, Koot HM, Alink LRA. Predicting growth curves of early childhood externalizing problems: differential susceptibility of children with difficult temperament. Journal of Abnormal Child Psychology. 2009;37(5):625–636. doi: 10.1007/s10802-009-9298-0. http://doi.org/10.1007/s10802-009-9298-0. [DOI] [PubMed] [Google Scholar]
- Miller R, Plessow F. Transformation techniques for cross-sectional and longitudinal endocrine data: application to salivary cortisol concentrations. Psychoneuroendocrinology. 2013;38(6):941–946. doi: 10.1016/j.psyneuen.2012.09.013. http://doi.org/10.1016/j.psyneuen.2012.09.013. [DOI] [PubMed] [Google Scholar]
- Okun ML, Krafty RT, Buysse DJ, Monk TH, Reynolds CF, Begley A, Hall M. What constitutes too long of a delay? Determining the cortisol awakening response (CAR) using self-report and PSG-assessed wake time. Psychoneuroendocrinology. 2010;35(3):460–468. doi: 10.1016/j.psyneuen.2009.08.017. http://doi.org/10.1016/j.psyneuen.2009.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesonen A-K, Kajantie E, Heinonen K, Pyhälä R, Lahti J, Jones A, Räikkönen K. Sex-specific associations between sleep problems and hypothalamic–pituitary– adrenocortical axis activity in children. Psychoneuroendocrinology. 2012;37(2):238–248. doi: 10.1016/j.psyneuen.2011.06.008. http://doi.org/10.1016/j.psyneuen.2011.06.008. [DOI] [PubMed] [Google Scholar]
- Platje E, Jansen LMC, Raine A, Branje SJT, Doreleijers TAH, de Vries-Bouw M, Vermeiren RRJM. Longitudinal associations in adolescence between cortisol and persistent aggressive or rule-breaking behavior. Biological Psychology. 2013 doi: 10.1016/j.biopsycho.2013.01.002. http://doi.org/10.1016/j.biopsycho.2013.01.002. [DOI] [PubMed] [Google Scholar]
- Resnick HS, Kilpatrick DG, Dansky BS, Saunders BE, Best CL. Prevalence of civilian trauma and posttraumatic stress disorder in a representative national sample of women. Journal of Consulting and Clinical Psychology. 1993;61(6):984–991. doi: 10.1037//0022-006x.61.6.984. [DOI] [PubMed] [Google Scholar]
- Rohleder N, Otto B, Wolf JM, Klose J, Kirschbaum C, Enck P, Klosterhalfen S. Sex-specific adaptation of endocrine and inflammatory responses to repeated nauseogenic body rotation. Psychoneuroendocrinology. 2006;31(2):226–236. doi: 10.1016/j.psyneuen.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Rudolph KD, Troop-Gordon W, Granger DA. Peer victimization and aggression: moderation by individual differences in salivary cortisol and alpha-amylase. Journal of Abnormal Child Psychology. 2010;38(6):843–856. doi: 10.1007/s10802-010-9412-3. http://doi.org/10.1007/s10802-010-9412-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruttle PL, Shirtcliff EA, Serbin LA, Fisher DB-D, Stack DM, Schwartzman AE. Disentangling psychobiological mechanisms underlying internalizing and externalizing behaviors in youth: longitudinal and concurrent associations with cortisol. Hormones and Behavior. 2011;59(1):123–132. doi: 10.1016/j.yhbeh.2010.10.015. http://doi.org/10.1016/j.yhbeh.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selya AS, Rose JS, Dierker LC, Hedeker D, Mermelstein RJ. A Practical Guide to Calculating Cohen’s f2, a Measure of Local Effect Size, from PROC MIXED. Frontiers in Psychology. 2012;3 doi: 10.3389/fpsyg.2012.00111. http://doi.org/10.3389/fpsyg.2012.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya I, Nagamitsu S, Okamura H, Ozono S, Chiba H, Ohya T, Matsuishi T. High correlation between salivary cortisol awakening response and the psychometric profiles of healthy children. BioPsychoSocial Medicine. 2014;8(1):9. doi: 10.1186/1751-0759-8-9. http://doi.org/10.1186/1751-0759-8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirtcliff EA, Vitacco MJ, Graf AR, Gostisha AJ, Merz JL, Zahn-Waxler C. Neurobiology of empathy and callousness: implications for the development of antisocial behavior. Behavioral Sciences & the Law. 2009;27(2):137–171. doi: 10.1002/bsl.862. http://doi.org/10.1002/bsl.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H, Belden AC, Spitznagel E, Dietrich R, Luby JL. Blunted stress cortisol reactivity and failure to acclimate to familiar stress in depressed and subsyndromal children. Psychiatry Research. 2013 doi: 10.1016/j.psychres.2013.06.038. http://doi.org/10.1016/j.psychres.2013.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Goozen SH, Matthys W, Cohen-Kettenis PT, Gispen-de Wied C, Wiegant VM, van Engeland H. Salivary cortisol and cardiovascular activity during stress in oppositional-defiant disorder boys and normal controls. Biological Psychiatry. 1998;43(7):531–539. doi: 10.1016/S0006-3223(97)00253-9. http://doi.org/10.1016/S0006-3223(97)00253-9. [DOI] [PubMed] [Google Scholar]
- Wright CE, Steptoe A. Subjective socioeconomic position, gender and cortisol responses to waking in an elderly population. Psychoneuroendocrinology. 2005;30(6):582–590. doi: 10.1016/j.psyneuen.2005.01.007. http://doi.org/10.1016/j.psyneuen.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Young R, Sweeting H, West P. Associations between DSM-IV diagnosis, psychiatric symptoms and morning cortisol levels in a community sample of adolescents. Social Psychiatry and Psychiatric Epidemiology. 2012;47(5):723–733. doi: 10.1007/s00127-011-0374-8. http://doi.org/10.1007/s00127-011-0374-8. [DOI] [PMC free article] [PubMed] [Google Scholar]