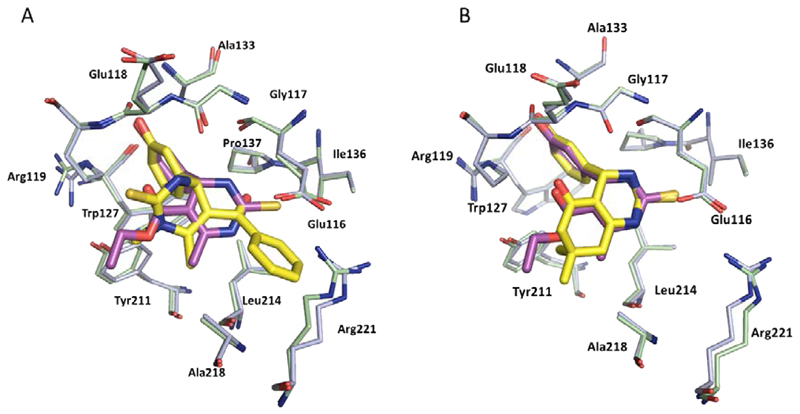
Figure 5.
A) Overlay of Monastrol (1) (magenta, PDB 1X88, light blue ADP-Eg5) and (R)-Mon-97, 2(R) (yellow, PDB 2IEH, pale green ADP-Eg5) showing the ‘flipped’ binding orientation. Hydrogen bonds between the phenolic OH of 2(R) with the backbone of Glu118 (2.6Å) and a weaker interaction with the side chain of Arg119 (3.4Å) analogous to those formed by the phenolic OH of monastrol exist but are not shown. An additional hydrogen bond between the NH of the dihydropyrimidinethione core and Gly117 (2.6Å) is also formed; B) Overlay of Monastrol (1) (magenta, PDB 1X88, light blue ADP-Eg5) and (S)-dimethylenastron (4) (yellow, PDB 2X7D, pale green ADP-Eg5). The two ligands bind in the same orientation and hydrogen bonds to the protein are identical.