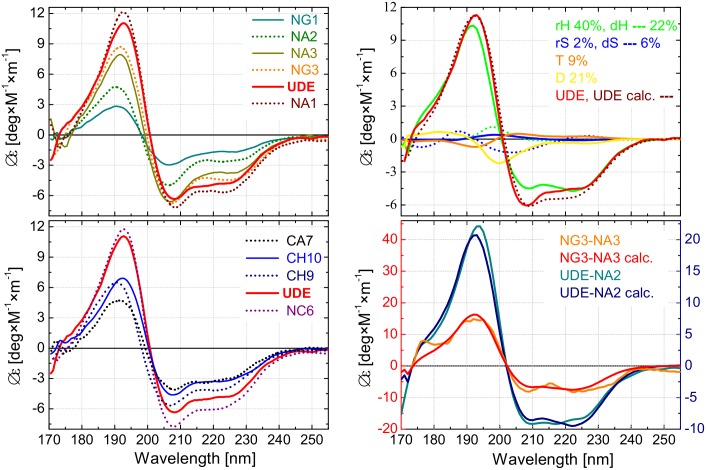
Fig 4. Secondary structure content determination of UDE and its fragments using VUVCD and SELCON3 program.
(A) Vacuum-ultraviolet circular dichroism (Δε) spectra of the UDE protein and its nine truncated fragments measured over the wavelength region of λ = 170–255 nm. The spectra are sorted into two panels for better visibility and spectra of UDE (red) is shown in both panels for reference (B) Decomposition of the CD spectra of UDE and its selected fragments using six secondary structure components; regular/distorted α-helix (rH/dH), regular/distorted β-strand (rS/dS), turn (T), and disordered structure (D). Upper panel: CD spectrum of UDE as measured and as fitted using the six components [22–24]. Spectra of the components are also plotted with magnitudes proportional to their ratios in the full-length protein. Lower Panel: Difference spectra corresponding to UDE-NA2 and NG3-NA3 together with the fittings based on the spectra of the six basic components.