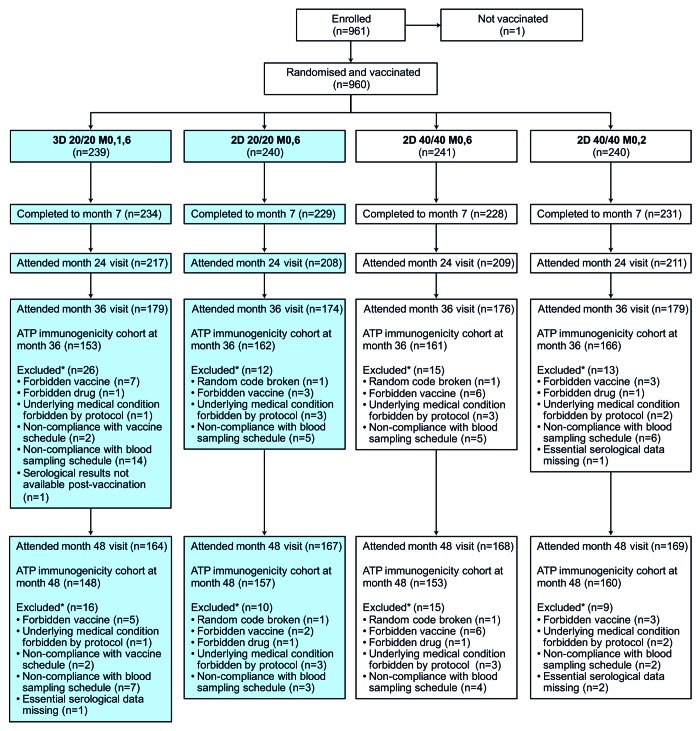
Figure 1. Flow of participants through the trial. 2D, 2-dose schedule; 3D, 3-dose schedule; 20/20, 20 μg each of HPV-16 and -18 L1 virus-like particles; 40/40, 40 μg each of HPV-16 and -18 L1 virus-like particles; ATP, according-to-protocol; M, month. This article focuses on subjects randomized to the 3D 20/20 M0,1,6 group and the 2D 20/20 M0,6 group (shaded boxes). Disposition data are also shown for subjects enrolled in other study groups (2D 40/40 M0,6 and 2D 40/40 M0,2) for completeness.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.