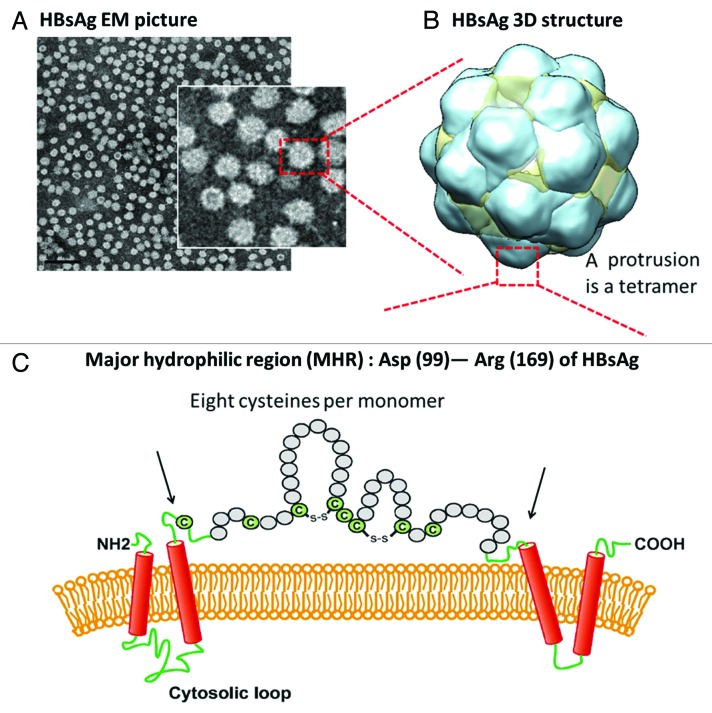
Figure 1. Recombinant cysteine-rich HBsAg, the active component of HBV vaccines, is a protein self-assembled into spherical particles upon expression with proper lipids. (A) Transmission electron-microscopy (TEM) obtained with CHO-derived HBsAg. Scale bar = 100 nm. (B) Three-dimensional octahedral HBsAg particle structure (yeast-derived HBsAg) with reconstructed cryo TEM data (adopted from Mulder et al.)5 (C) Major hydrophilic region (MHR), Asp99 to Arg169, in each monomer is illustrated with 8 cysteines per monomer. Therefore, a total of 32 cysteines are present in each protrusion which comprises of a tetrameric HBsAg MHR. It is conceivable that the disulfide pairing in MHR is complex. The presence of cysteines and formation of disulfides were shown to be crucial in virions for budding and viral post-replication transport.9,10 These disulfides are also important in the HBsAg VLPs in maintaining the virion-like epitopes,8 Therefore, making stable and cross-linked HBsAg antigens is the prime goal during vaccine bioprocess.19

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.