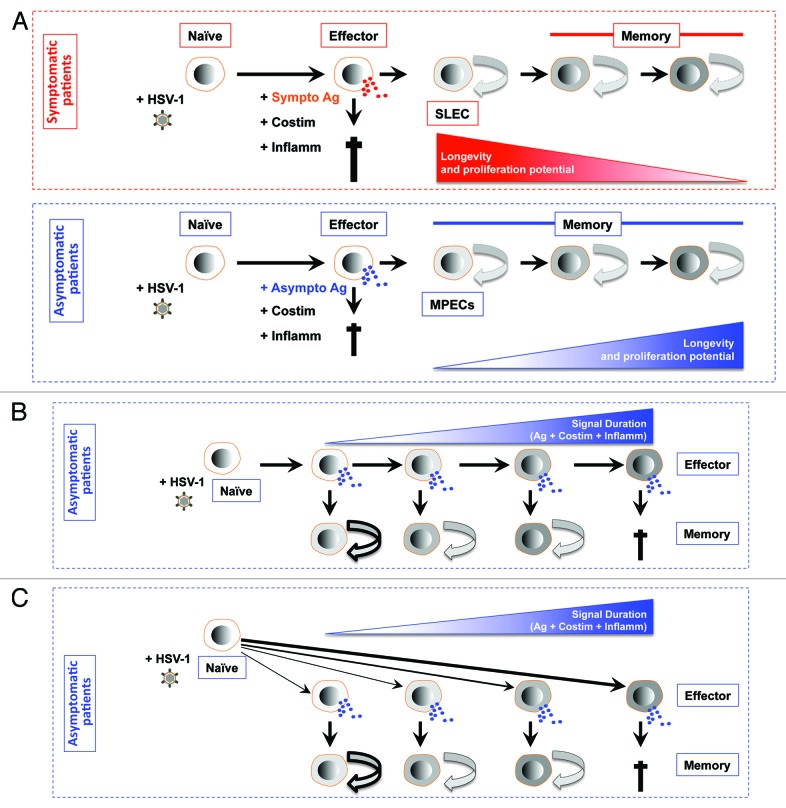
Figure 3. Models for generating diverse differentiated states of effector and memory CD8+ T cells following stimulation by symptomatic vs. asymptomatic epitopes. (A) First working model of de-differentiation for generating CD8+ T cell diversity proposes that soon after symptomatic or asymptomatic activation daughter naïve CD8+ cells become fully differentiated and functional by producing cytokines and exhibiting cytotoxic activity. The majority of effector CD8+ T cell then die. While a minority of asymptomatic epitope specific CD8+ T cells will de-differentiate into MPEC and long-lived memory CD8+ T cells. (B) Second working linear model for generating CD8+ T cell diversity proposes that degree of effector cell differentiation is regulated by the duration of exposure of daughter cells to symptomatic and asymptomatic epitopes following repetitive reactivation of HSV-2 from latency. The majority of terminally differentiated effector CD8+ T cell will die following cumulative encounter with the virus. While a minority of asymptomatic epitope specific CD8+ T cells which did not reach the end stage of differentiation will develop into MPEC and long-lived memory CD8+ T cells. The memory CD8+ T cell phenotypes vary according to the differentiation state of the effector cells from which they descended; curved arrows with bold, thin, or dashed lines indicate a high, medium, or low degree of proliferative potential and longevity. (C) Third working divergent model for generating CD8+ T cell diversity is similar to B, except that the strength of symptomatic vs. asymptomatic signal (instead of the cumulative symptomatic vs. asymptomatic stimulation) will determine the degree of effector cell differentiation and formation MPEC and long-lived memory CD8+ T cells.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.