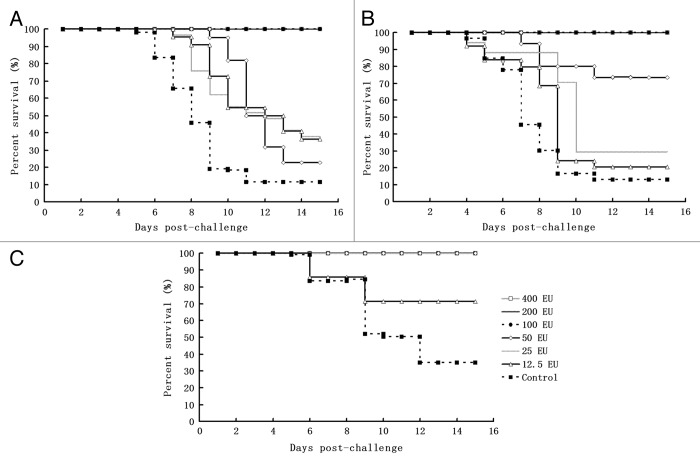
Figure 3. The immune protective efficacy of the candidate inactivated CA16 vaccine against infection with different challenge viruses in a neonatal passive immunity mouse model. Three groups of suckling mice (24–48 h after birth) obtained from the immunized maternal mice of each dosage group after the secondary immunization were injected intracerebrally with either CA16 virus G-20, MA154, or G10 (104CCID50 per mouse), and the control group from the parents was injected with the 20 µl Al(OH)3 adjuvant. Subsequently, the survival rates were recorded daily after infection for a period of 15 d. (A) G20 challenge group: 400 EU x 2n (n = 11–12), 200 EU × 2n (n = 12–13), 100 EU × 2n (n = 10–12), 50 EU × 2n (n = 12–15), 25 EU × 2n (n = 11–12), 12.5 EU × 2n (n = 12–13), n.c (negative control) × 2n (n = 11–12). 2n indicates the number (n) of mice in 2 replicates of a dose group. (B) G10 challenge group: 400 EU × 2n (n = 12–13), 200 EU × 2n (n = 10–11), 100 EU × 2n (n = 10–11), 50 EU × 2n (n = 12–15), 25 EU × 2n (n = 11–16), 12.5 EU × 2n (n = 13–15), n.c × 2n (n = 10–12). (C) MA154 challenge group: 400 EU × 2n (n = 10–11), 200 EU × 2n (n = 12–13), 100 EU × 2n (n = 10–13), 50 EU × 2n (n = 12–13), 25 EU × 2n (n = 11–15), 12.5 EU × 2n (n = 10–13), n.c × 2n (n = 12–14)

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.