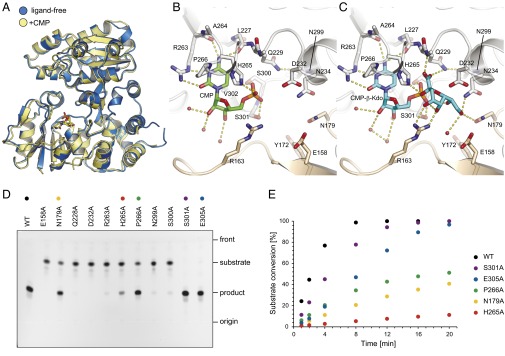
Fig. 6.
CMP binding to WbbB2–401 and in vitro activity of WbbB2–401 and site-directed mutants. (A) Superposition of ligand-free (blue) and CMP bound (yellow) of WbbB2–401. Local movement in the C-terminal α/β domain accompanies CMP binding; note that this movement occurs at the dimeric interface. (B) Details of CMP binding site. (C) Model of CMP-β-Kdo in the binding site. The ligand was placed by superimposing the CMP portion of the molecule, then positioning the sugar ring by torsions around the P–O and O–C2 bonds. Potential hydrogen bonds are shown in dashed lines. (D) The reaction mixtures were incubated for 20 min and analyzed by TLC. Of six mutants showing little or no activity on TLC, E158A and D232 were completely inactive, whereas others retained residual activity (<3% conversion after 20 min). (E) Time course of Kdo-transferase reaction. The percentage of conversion was determined using LC-MS by integrating the peak areas of the substrate and the product in the merged extracted ion chromatograms.