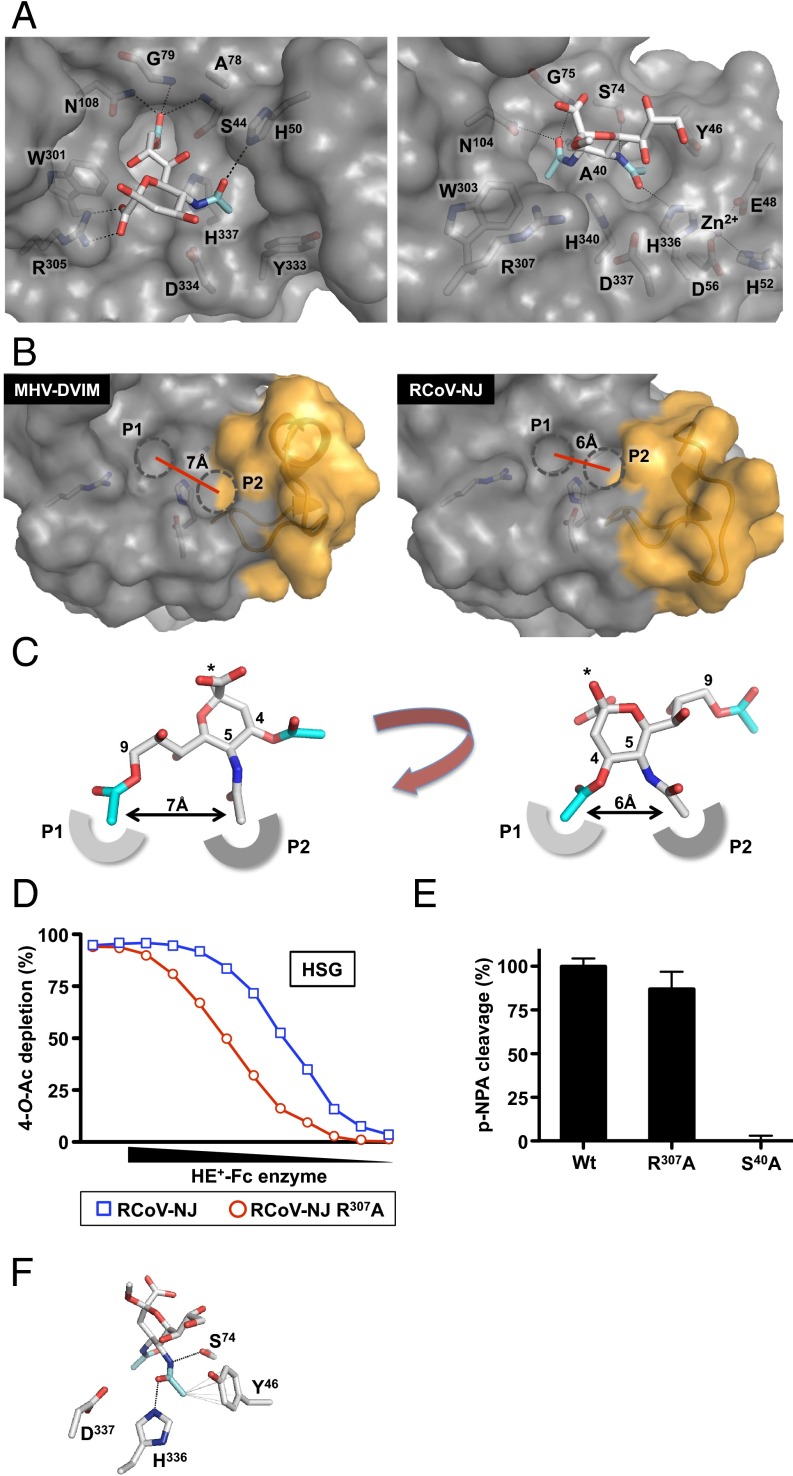
Fig. 3.
(A) Surface representation of the MHV-DVIM HE (Left) and RCoV-NJ HE (Right) catalytic sites in complex with 9-O-Ac-Sia [docked with Autodock4 (55)] and 4-N-Ac-Sia (crystal complex), respectively. (B) Surface representation of the catalytic sites of MHV-DVIM HE (Left) and RCoV-NJ HE (Right). The active-site Ser44 in MHV-DVIM HE already adopts the “active” rotamer observed in HEF (18, 39, 40); For RCoV-NJ HE crystallized as an inactive Ser-to-Ala mutant, a Ser side chain with active rotamer was introduced using COOT. The P1 and P2 pockets are highlighted by dashed circles; approximate distances between pockets, as measured from the centers, are indicated. (C) Binding topology of αNeu4,5,9Ac3 in type I (Left) and type II (Right) esterases. The P1 and P2 pockets accommodating the O- and N-acetyl moieties are shown schematically. αNeu4,5,9Ac3 is shown in stick representation and colored as in Fig. 2C. Asterisks indicate the position of the O2 atom through which Sias are glycosidically linked. The distances between 5-N- and 9-O- or 4-O-Ac methyl groups are shown. (D) RCoV-NJ HE Arg307 is not essential for sialate-4-O-acetylesterase activity. Ser40Ala is a catalytically inactive mutant. Receptor destruction was assessed as in Fig. 1B. For a comparison with type I HEs, see Fig. 2E. (E) Arg307Ala substitution in RCoV-NJ HE does not affect activity toward the synthetic substrate pNPA. Enzymatic activity shown as percentage of wild-type activity. (F) Hydrogen bonding of the sialate-5-N-acyl carbonyl oxygen and amide nitrogen with RCoV-NJ HE Ser74 and His336, respectively, as observed in the crystal complex, indicated as in Fig. 2C. Hydrophobic contacts between Tyr46 and the Sia-5-N-acyl methyl group are shown as thin gray lines.