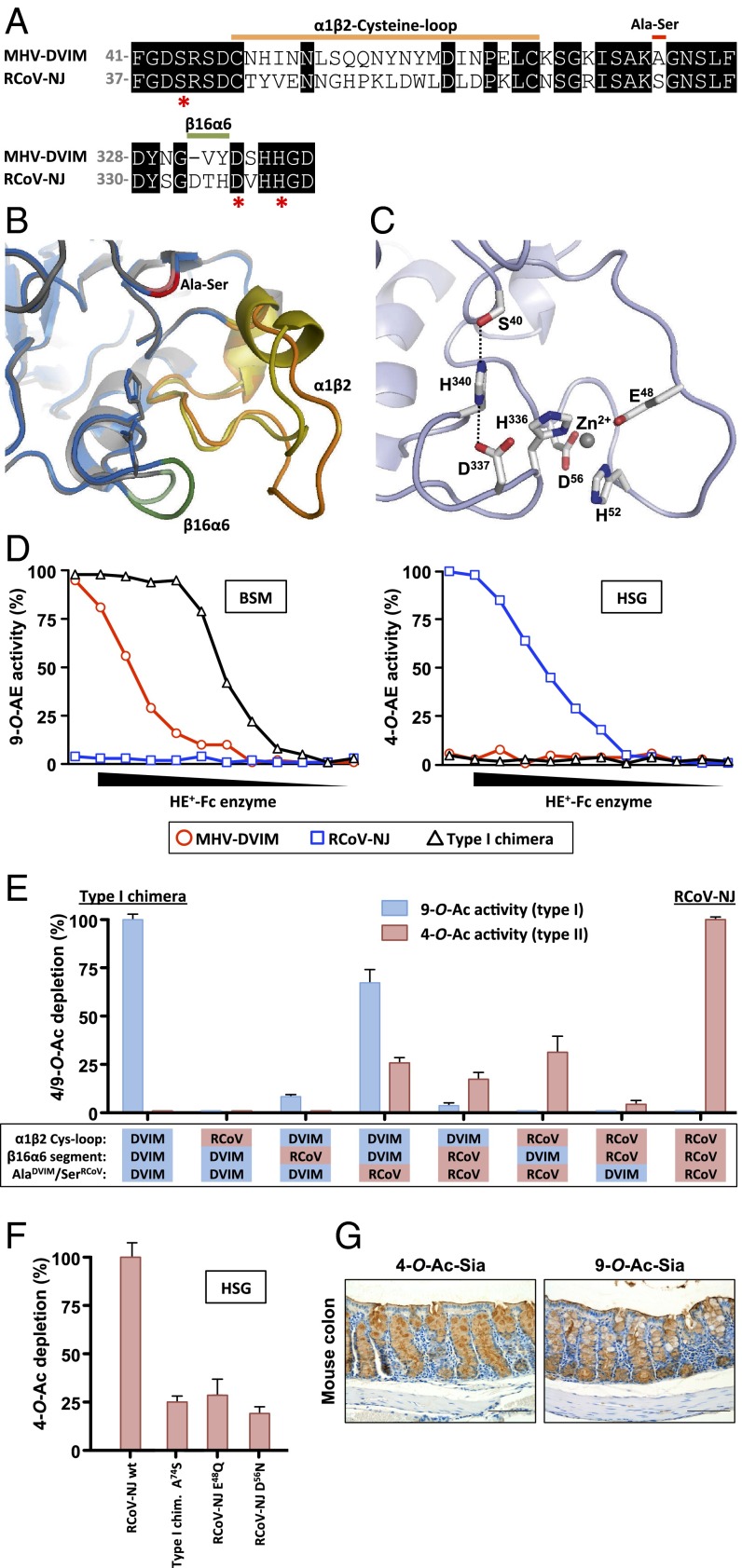
Fig. 4.
(A) Partial sequence alignment of MHV-DVIM and RCoV-NJ HE, highlighting consistent differences between type I and type II HEs (Fig. S1). Aligned sequences, with residue numbering presented Left and Right, cover the α1β2-cysteine-loop, the β2α3 segment (single Ala78Ser substitution), and the β16α6 segment. Catalytic residues (Ser, Asp, His) are marked with asterisks. (B) Overlay of cartoon representations of the active-site regions of MHV-DVIM HE (gray) and RCoV-NJ HE (blue). Side chains of catalytic triad residues are depicted as sticks. The three type I/II distinctive elements are colored as in A. (C) Cartoon representation of the novel metal-binding site near the RCoV-NJ HE active site, formed by Glu48, His52, Asp56, and His336. The catalytic triad is shown for reference. Side chains are depicted as sticks, the Zn2+ ion as a gray sphere. (D) A type II HE converted into a type I enzyme. An RCoV HE-based chimera with all three type I/II distinctive elements replaced by those of MHV-DVIM displays strict sialate-9-O-acetylesterase activity. The enzyme activity of the recombinant protein (“Type I chimera”) was compared with that of the parental proteins (MHV-DVIM and RCoV-NJ HE) on BSM (Left) and HSG (Right). Cleavage of 9-O- and 4-O-Ac-Sias was assessed as in Fig. 1B, but now starting at 10 ng/µL. (E) Contribution of the three type I/II distinctive elements to esterase activity and substrate specificity. The type I chimera was subjected to mutational analysis entailing systematic reintroduction of RCoV-NJ segments. Esterase activities of chimeric proteins toward 9-O-Ac- (blue bars) and 4-O-Ac-Sias (red bars) were determined in twofold dilution series as in Fig. 1B. Data are shown as percentages of specific esterase activity, calculated at 50% receptor depletion, relative to that of the type I chimera (for 9-O-Ac-Sia) or of wild-type RCoV-NJ HE (for 4-O-Ac-Sia). The error bars represent the SD over six measurements (two biological replicates, each of which performed in technical triplicates). (F) The type II esterase metal-binding site is required for full 4-O-AE activity. Note that disruption of metal binding by either Glu48Gln or Asp56Asn substitution reduces sialate-4-O-acetylesterase activity by 75% (comparable to the amount of type II activity conferred by the Ala74Ser substitution alone). Enzymatic activity measured as in Fig. 1B and presented as in Fig. 4E. (G) 4-O- and 9-O-Ac-Sias are abundantly expressed in the mouse colon. Paraffin-embedded mouse colon tissue sections were stained for 4-O-Ac-Sia with MHV-S HE0-Fc, and for 9-O-Ac-Sia with PToV-P4 HE0-Fc.