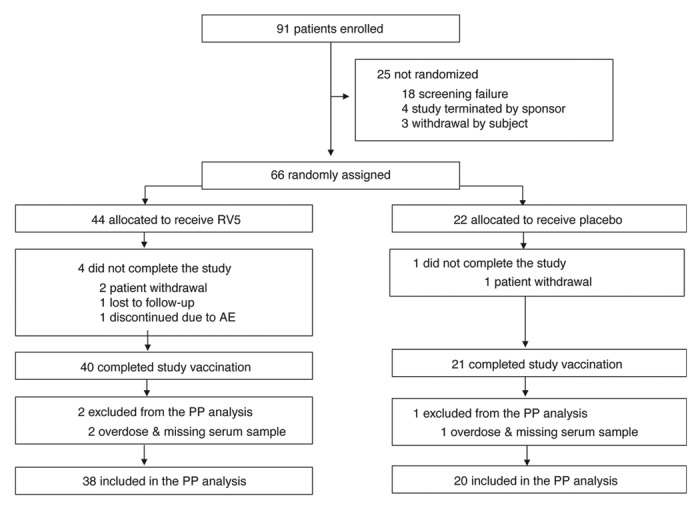
Figure 1. Study disposition. AE, adverse event; PP, per-protocol; RV5, pentavalent rotavirus vaccine, RotaTeq®. Note: Overdose was defined as receiving more than 1 dose within a 12-d period. Missing serum sample refers to a subject missing either baseline or postdose 3 serum sample for immunogenicity testing.
