Figure 6. The synaptic interplay of mGlu4, mGluR5 and calcium dependent vasoactive signaling.
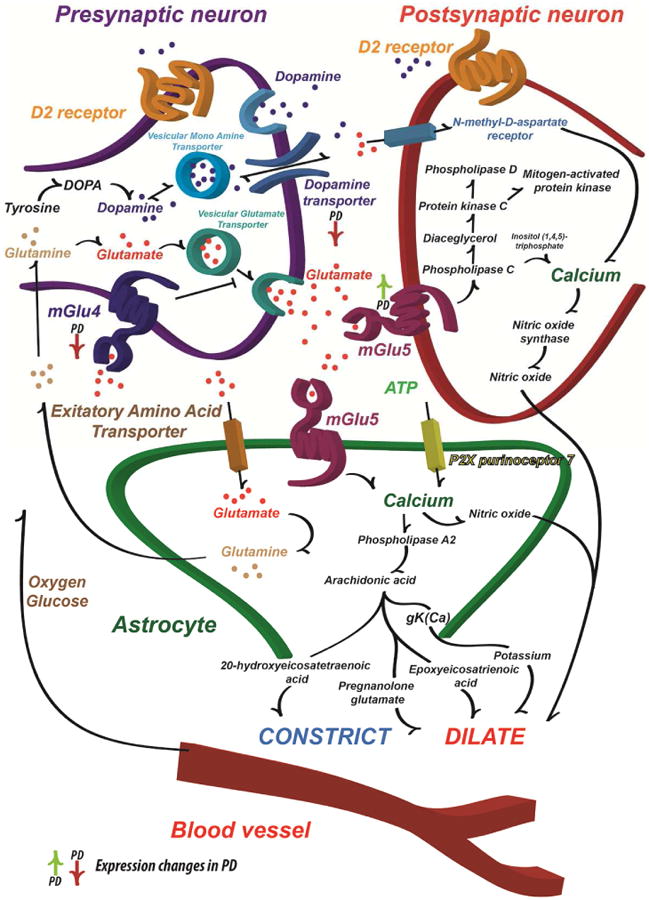
Schematic illustration of the major DA and Glu synaptic effects and how these may influence cerebral blood flow. The primary pathophysiological change that leads to the motor symptoms of PD is the loss of dopaminergic neurons in the substantia nigra pars compacta that modulates the function of the striatum and other basal ganglia structures. The L-DOPA is considered the standard of care for providing symptomatic relief for PD patients. However, long-term L-DOPA treatment leads to the appearance of motor complications (Ahlskog and Muenter, 2001). On the other hand, the excessive Glu signaling in the basal ganglia is one of the hallmarks of the progression of PD. The mGlu (especially mGlu4 and mGlu5) modulators were considered to be one of the best potential drug candidates since the striatum also sends direct projections to the substantia nigra pars compacta, which leads to a parallel increase in Glu release, GABA signaling and contributes to the progressive neuronal degeneration also via an excitotoxic mechanism (Gasparini et al., 2013). The hyperactive Glu stimulus demands energy and couples the hyperactivation tightly with blood flow (Lauritzen et al., 2012). It has been suggested that the astrocytes are able to detect the Glu driven synaptic activity and thus modulate the local blood flow (Attwell et al., 2010). In addition, ATP and its derivative adenosine modulates the blood flow (Latini and Pedata, 2001) as does DA acting on both astrocytes and blood vessels (Choi et al., 2006). Together with dopamine transporter, the synaptic contacts possess a variety of neurotransmitter receptors, including both ionotropic and metabotropic glutamate receptors (Beaulieu and Gainetdinov, 2011). Activation of Glu receptors by synaptically released Glu is known to lead to transient and graded increases in intracellular Ca2+ concentration (Marambaud et al., 2009). Since the Glu signaling is hyperactive in PD, it is assumed that the inhibition of Glu signaling should decrease the PD symptoms, the synaptic activity and eventually lead to decrease of the local blood flow due to decreased energy demand and Ca2+ signaling in pathological site (Attwell et al., 2010; Lauritzen et al., 2012).
