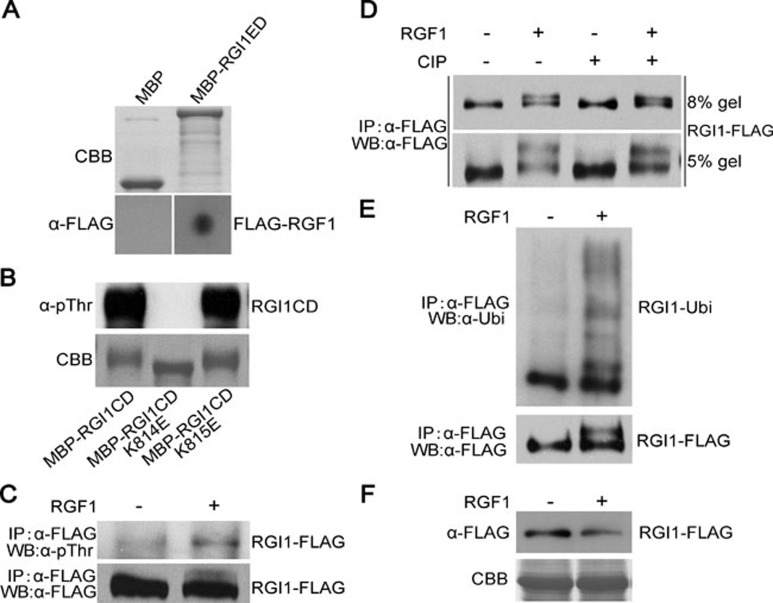
Figure 6.
RGI1 interacts with RGF1, and RGF1 induces the phosphorylation and poly-ubiquitination of RGI1. (A) Dot blotting assay showing that MBP-RGI1ED, but not MBP, can interact with FLAG-RGF1. Top panel shows Commassie blue-stained 100 ng column-purified MBP and MBP-RGI1ED after SDS-PAGE. Bottom panel shows that immobilized 100 ng MBP-RGI1ED but not MBP can interact with the FLAG-RGF1 probe. An anti-FLAG antibody was used for the immunoblotting assay. (B) E. coli-expressed MBP-RGI1CD showing strong autophosphorylation signal. Mutation of K814E but not K815E abolished its autophosphorylation. (C) A transgenic line harboring 35S::RGI1-FLAG shows induced phosphorylation of RGI1-FLAG after the treatment of 20 μM RGF1 for 30 min in a 1/2 MS medium liquid culture. (D) The 35S::RGI1-FLAG transgenic line was treated with or without 20 μM RGF1 for 30 min. RGI1-FLAG was immunoprecipitated and treated with or without CIP. The proteins were then subjected to western blot analysis using anti-FLAG antibody. (E) RGF1 treatment can induce the poly-ubiquitination of RGI1. Top panel shows the immunoblotting result using an anti-ubiquitin antibody. Bottom panel is the immunoblotting result using an anti-FLAG antibody. (F) RGF1 treatment can significantly reduce the protein level of RGI1. Three-week-old Arabidopsis rosette leaves were treated with or without 20 μM RGF1 for 30 min before total protein was extracted and analyzed by immunoblotting.