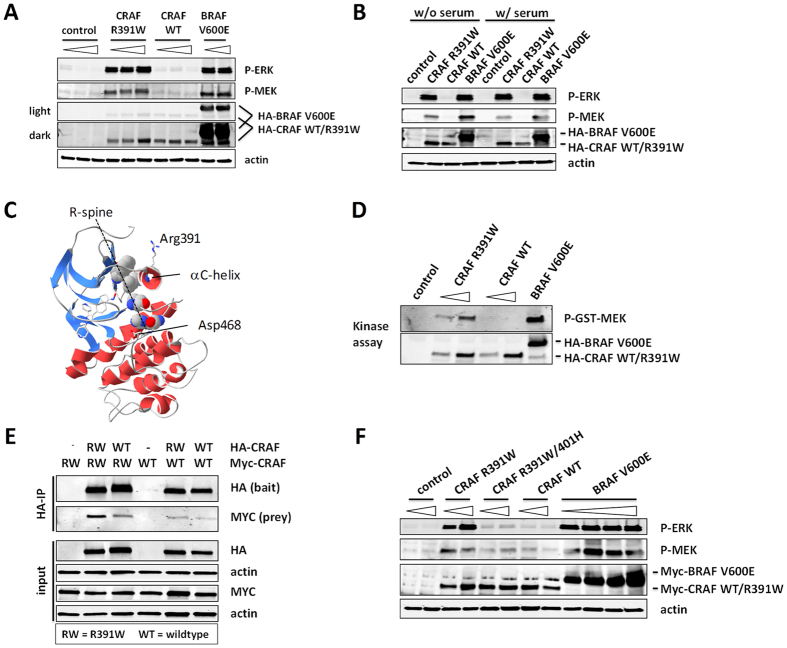
Figure 3. Engineered CRAF R391W expression reconstitutes MAP-kinase activation dependent on CRAF dimerization.
(A) CRAF R391W activates downstream MAP-kinase signaling. An empty vector control, CRAF R391W, CRAF wild type (WT), and BRAF V600E were transiently expressed in 293T cells and downstream MAP-kinase pathway activation was measured by Western blot after 48 h (p-ERK T202/Y204 and p-MEK S217/221). The N-terminally HA-tagged genes (pDS_HA vector) were transfected in increasing amounts (2, 4, 8 μg DNA per one well of a 6-well plate; 2 and 4 μg for BRAF V600E; as indicated by the gradient triangles). Expression of the transfected genes was tested with an anti-HA antibody. The relatively faint lower band in the HA-BRAF lane, which runs slightly faster than the HA-CRAF protein, appears to be a minor degrative product of the exogenously produced HA-BRAF protein. (B) MAP-kinase pathway activation by CRAF R391W does not depend on serum-derived growth factors. Experiment in (A) was repeated in the absence (w/o) and presence (w/) of serum. (C) Arg391 is located in the αC-helix of CRAF. Structure 3OMV from PDB database visualized with SwissPDB-Viewer48,49. (D) CRAF R391W shows increased kinase activity. The empty control vector (N-terminal HA-tag, pDS_HA, 8 μg DNA per 6cm plate), CRAF R391W (4 and 8 μg DNA), CRAF wild type (WT, 4 and 8 μg DNA), and BRAF V600E (4 μg DNA) were transiently transfected into 293T cells. Anti-HA immunoprecipitation and in vitro kinase assay were performed with GST-MEK as the substrate. Phosphorylation of GST-MEK by the precipitated kinases was detected by Western blot. (E) CRAF R391W shows increased homo-dimerization. HA- and Myc-tagged constructs of CRAF R391W (RW), CRAF wild type (WT), and the empty HA-vector were transiently transfected into 293T cells. Expression was checked by Western blot (input). The HA-tag was immuno-precipitated and co-precipitation of Myc-tagged proteins was checked by Western blot (HA-IP). (F) CRAF R391W activity depends on its dimerization state. The previously described R401H mutation was introduced into CRAF R391W to interfere with functional dimer formation. Downstream MAP-kinase activation of the different constructs was tested as in (A) (2 or 4 μg DNA transfected, 0.5, 1, 2, or 4 μg DNA of BRAF V600E).