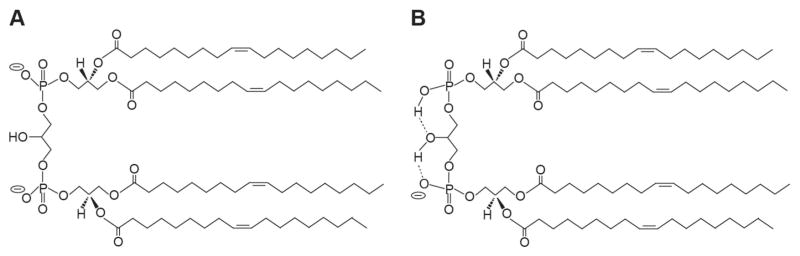
Fig. 1.
Cardiolipin structure. Chemical structures of tetraoleoyl cardiolipin (1′,3′-bis[1,2-dioleoyl-sn-glycero-3-phospho]-sn-glycerol) representing the two models of headgroup ionization state at physiological pH. A) Low pKa values of both phosphates. In this model, both phosphates ionize independently, each assuming proton dissociation behavior corresponding to the first protolysis step of phosphoric acid. Thus the headgroup exists as a dianion at physiological pH. B) Disparate pKa values of the two phosphates. In this model, the phosphates form a bicyclic ring structure above the two sn-1 acyl chains, which is stabilized by resonance (not shown). In this acid-anion structure, the secondary hydroxyl of the central glycerol mediates hydrogen bonding interactions (dashed lines) that stabilize the protonated form of one phosphate and the headgroup exists as a monoanion at physiological pH.