Fig. 8.
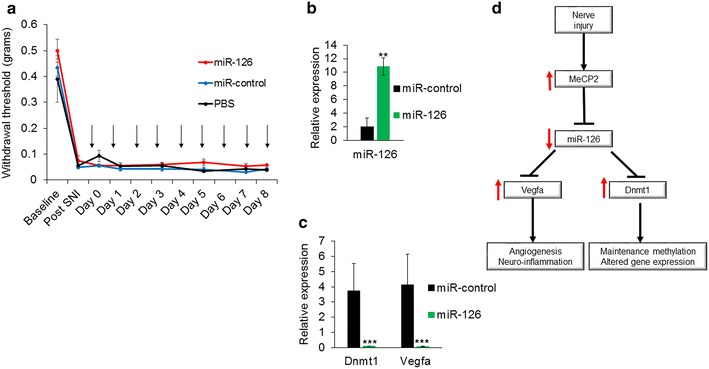
Administration of exogenous miR-126 decreased Dnmt1 and Vegfa expression in vivo but did not alter pain sensitivity. a Mechanical sensitivity measured by von Frey filaments showed that intrathecal delivery of miR-126 did not alter the paw withdrawal threshold in SNI model mice. Arrows indicate daily intrathecal injections with 2 nmol miR-126 or control miRNA via catheter (n = 5 for miR-126 and miR-control injected mice, n = 3 for PBS injected mice). b Confirmation of miR-126 delivery to DRG. A qPCR performed using DRG collected from mice injected with miR-126 or control miRNA showed an increase in miR-126 in mice that received miR-126 compared to miR-control injected mice indicating successful delivery. c Relative expression of Dnmt1 and Vegfa mRNA in the DRG of miR-126 and control injected mice. Increased miR-126 decreased the expression of endogenous Dnmt1 and Vegfa compared to miR-control injected mice. Significance determined using unpaired Student’s t test, p value, **<0.01, ***<0.001 (n = 5 for miR-126 and miR-control injected mice, n = 3 for PBS injected mice). d Schematic representation of nerve injury-induced alterations of MeCP2 and downstream gene expression changes. SNI induced enriched binding of MeCP2 to miR-126 locus resulting in repression of miR-126. Lower miR-126 leads to increased expression of its two target genes Vegfa and Dnmt1. Vegfa could contribute to the progression of pain pathology by modulating angiogenesis and neuro-inflammation. Dnmt1 propagate established methylation patterns during cellular division by recognizing and copying parent strand methylation at symmetrical CG dinucleotides to the newly synthesized daughter strand and its increase after SNI can alter or maintain methylation, and may contribute to gene expression changes ensuing SNI
