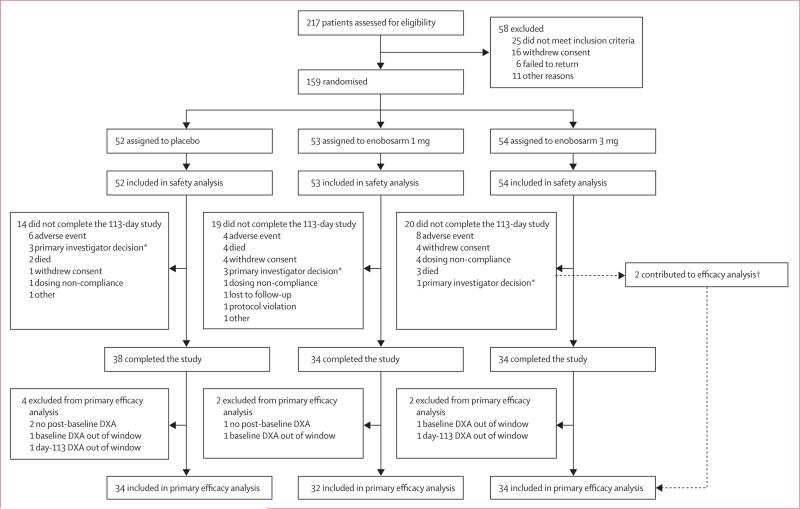
Figure 1. Trial profile.
Because of the prevalence of end-stage cancer in this study, 27 deaths occurred; nine discontinued because of death, 16 died after study discontinuation, and two died after study completion. *Removals were done before unmasking of primary investigators. †Two participants in the enobosarm 3 mg group were included in the efficacy analysis but did not complete the trial (one withdrew consent, one because of non-compliance).