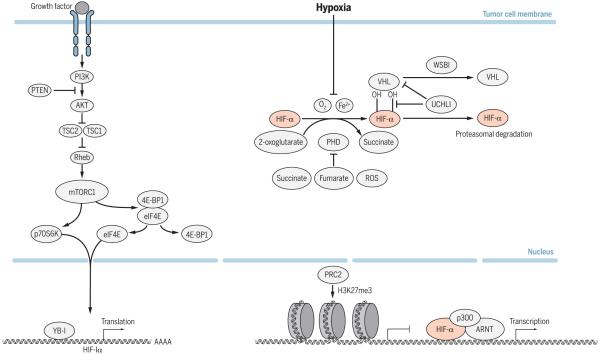
Fig. 1. Mechanisms of HIF-1 and HIF-2 activation in tumor cells.
Hypoxia is a common mechanism of HIF activation in cancer. Under normoxic conditions, PHD enzymes (also called EGLN 1–3) utilize oxygen as a substrate to hydroxylate key proline residues located within the HIF-α subunit. This hydroxylation event mediates pVHL binding and subsequent ubiquitination and degradation by the 26S proteasome. Under conditions of hypoxia or loss of pVHL, HIF-α is stabilized and translocates to the nucleus, where it heterodimerizes with ARNT and binds to hypoxia response elements (HREs) within regulatory regions of target genes. The HIF heterodimer activates gene expression at these sites upon cofactor (p300/CBP) recruitment. PRC2-mediated histone methylation can inhibit the activation of HIF target genes involved in metastasis. HIF activity can also be induced in tumor cells through mechanisms that enhance HIF-α production (TORC1, YB-1) or prevent its degradation (UCHL1, WSB1).