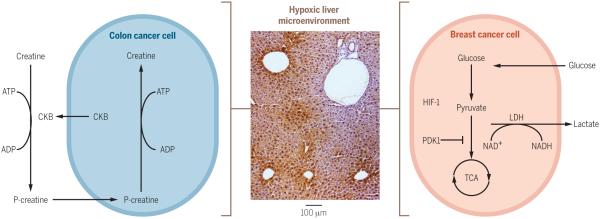
Fig. 3. Mechanisms of metabolic reprogramming in liver metastasis.
The liver microenvironment is hypoxic. Pimonidazole staining (brown) demonstrates areas of hypoxia in the liver (middle panel). Metastatic tumor cells enter hypoxic regions of the liver and must metabolically adapt to survive the metabolic stress associated with hypoxia. Disseminated breast cancer cells (right) metabolically adapt by increasing the expression of pyruvate dehydrogenase kinase 1 (PDK1) and promoting glycolytic reprogramming. Disseminated colon cancer cells (left) metabolically adapt by increasing the expression and secretion of creatine kinase brain-type (CKB). This enzyme controls the amount of rapidly mobilized high-energy phosphates by catalyzing the transfer of a high-energy phosphate group from ATP to the metabolite creatine, producing phosphocreatine. Under conditions of metabolic stress, such as hypoxia, tumor cells utilize phosphocreatine stores as a source of high-energy phosphate that can be transferred to ADP to generate ATP.