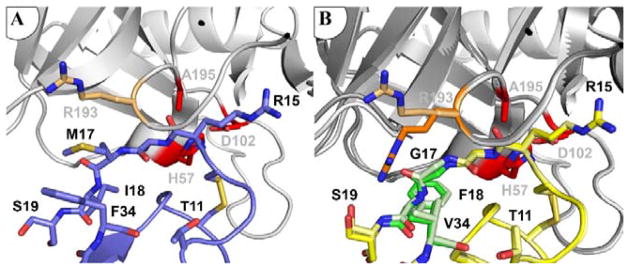
Figure 5. Mesotrypsin Arg-193 adopts multiple conformations in the complex with APPIM17G/I18F/F34V.
(A) Arg-193 conformation is constrained by interaction with APPI Met-17 in the mesotrypsin complex with APPIWT. Mesotrypsin is shown in gray with catalytic triad residues in red, and the Arg-193 side chain rendered as light orange sticks; APPIWT is shown in blue (PDB entry: 3L33). (B) In complex with APPIM17G/I18F/F34V, mesotrypsin Arg-193 is found in different conformations in the two copies of the structure in the asymmetric unit of the crystal, which here are shown superposed. Mesotrypsin is shown in gray, with catalytic triad residues in red; APPIM17G/I18F/F34V is shown in yellow, with mutated residues in green. The distinct ‘up’ and ‘down’ conformations of Arg-193 are illustrated in light and dark orange, respectively.