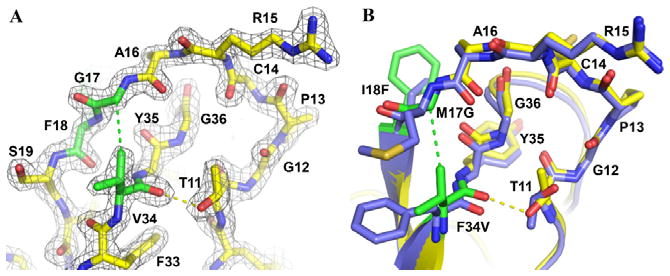
Figure 7. Enhanced intramolecular stabilizing features of APPIM17G/I18F/F34V.
The structure of APPIM17G/I18F/F34V bound to mesotrypsin reveals a new hydrophobic contact between Val-34 Cγ1 and Gly-17 Cα (green dashed line) and a new H-bond between Val-34 O and Thr-11 OH (yellow dashed line). (A) 2Fo-Fc density map is shown contoured at 2.0σ. APPIM17G/I18F/F34V is shown in yellow, with mutated residues in green. (B) APPIM17G/I18F/F34V is colored as above, superimposed upon APPIWT in blue to highlight small shifts in backbone positions. Thr-11 adopts a different rotamer conformation to facilitate formation of the H-bond.