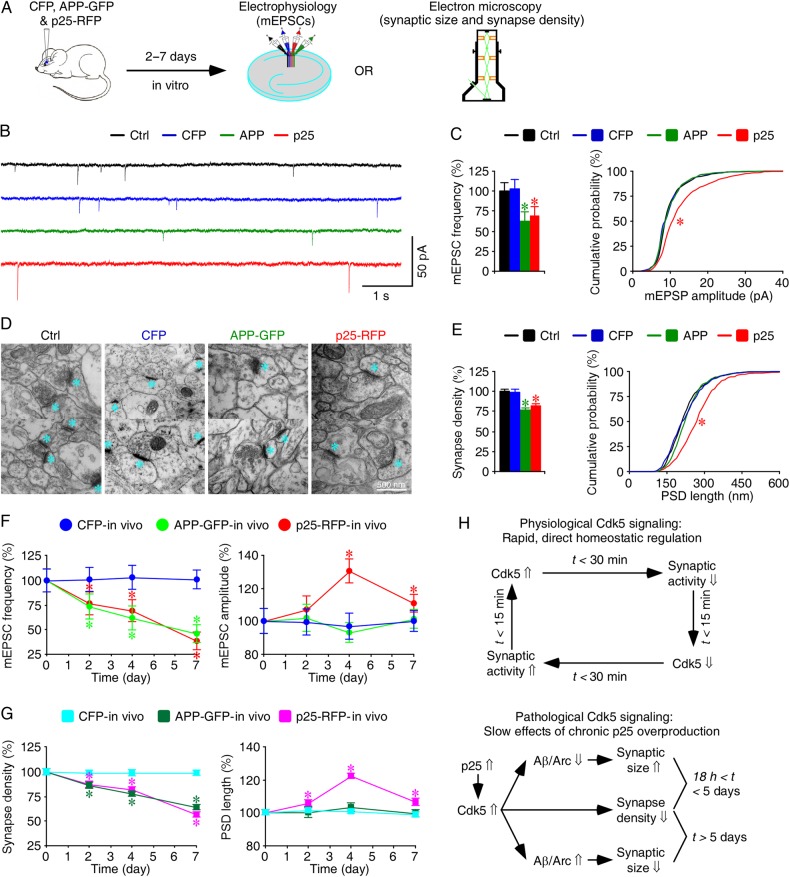
Figure 7.
Chronic overproduction of p25 induces Alzheimer-like synaptic alterations in intact brains. (A) Schematic drawing outlines in vivo experimental design. (B) Miniature EPSCs recorded simultaneously from nearby control nonexpressing CA1 neurons, CA1 neurons overexpressing CFP, APP-GFP, or p25-RFP in acute slices after 4 days of in vivo expression. (C) Average mEPSC frequencies of control nonexpressing CA1 neurons, CA1 neurons overexpressing CFP, APP-GFP, or p25-RFP (left), and cumulative distributions of mEPSC amplitudes of control nonexpressing CA1 neurons, CA1 neurons overexpressing CFP, APP-GFP, or p25-RFP (right) after 4 days of in vivo expression. (D) Electron microscopic images from hippocampal CA1 stratum radiatum regions of control rats, rats overexpressing CFP, APP-GFP, or p25-RFP after 4 days of in vivo expression. Cyan asterisks indicate individual synapses. (E) Average synaptic densities of control nonexpressing CA1 tissues, CA1 tissues overexpressing CFP, p25-RFP, or APP-GFP (left), and cumulative distributions of PSD lengths of control nonexpressing CA1 tissues, CA1 tissues overexpressing CFP, APP-GFP, or p25-RFP (right) after 4 days of in vivo expression. (F) (Left) Relative mEPSC frequency in control nonexpressing CA1 neurons, CA1 neurons overexpressing CFP, APP-GFP, or p25-RFP at different expression time. Value for the average mEPSC frequency of each neuron group after 2-day (CFP: 0.44 ± 0.05 Hz, n = 20, P = 0.77; APP: 0.32 ± 0.06 Hz, n = 20, P < 0.01; p25: 0.33 ± 0.05 Hz, n = 20, P < 0.05; Wilcoxon tests compared with Ctrl: 0.44 ± 0.05 Hz, n = 20), 4-day (CFP: 0.50 ± 0.06 Hz, n = 17, P = 0.62; APP: 0.30 ± 0.06 Hz, n = 17, P < 0.005; p25: 0.34 ± 0.05 Hz, n = 17, P < 0.05; Wilcoxon tests compared with Ctrl: 0.49 ± 0.05 Hz, n = 17), and 7-day (CFP: 0.59 ± 0.06 Hz, n = 18, P = 0.95; APP: 0.27 ± 0.05 Hz, n = 18, P < 0.01; p25: 0.23 ± 0.05 Hz, n = 18, P < 0.005; Wilcoxon tests compared with Ctrl: 0.59 ± 0.06 Hz, n = 18) in vivo overexpression. (Right) Relative mEPSC amplitude in control nonexpressing CA1 neurons, CA1 neurons overexpressing CFP, APP-GFP, or p25-RFP at different expression time. Values for the average mEPSC amplitude of each neuron group after 2-day (CFP: 9.21 ± 0.70 pA, n = 20, P = 0.94; APP: 9.44 ± 0.77 pA, n = 20, P = 0.85; p25: 9.88 ± 0.75 pA, n = 20, P = 0.19; Wilcoxon tests compared with Ctrl: 9.23 ± 0.69 pA, n = 20), 4-day (CFP: 8.82 ± 0.72 pA, n = 17, P = 0.65; APP: 8.48 ± 0.55 pA, n = 17, P = 0.41; p25: 12.64 ± 0.81 pA, n = 17, P < 0.005; Wilcoxon tests Ctrl: 9.07 ± 0.50 pA, n = 17), and 7-day (CFP: 9.46 ± 0.59 pA, n = 18, P = 0.91; APP: 9.57 ± 0.55 pA, n = 18, P = 0.40; p25: 10.45 ± 0.49 pA, n = 18, P < 0.05; Wilcoxon tests compared with Ctrl: 9.45 ± 0.62 pA, n = 18) in vivo overexpression. (G) (Left) Relative synapse density in control nonexpressing CA1 neurons, CA1 neurons overexpressing CFP, APP-GFP, or p25-RFP at different expression time. Values for the average synapse density, counted as synapses per 100 µm2, after 2-day (CFP: 12.4 ± 0.4, n = 50 ultrathin sections from 8 animals, P = 0.59; APP: 10.7 ± 0.4; n = 49 ultrathin sections from 8 animals, P < 0.005; p25: 10.9 ± 0.4, n = 50 ultrathin sections from 8 animals, P < 0.005; Mann–Whitney Rank Sum tests compared with Ctrl: 12.5 ± 0.4, n = 50 from 8 animals), 4-day (CFP: 12.4 ± 0.4, n = 50 ultrathin sections from 8 animals, P = 0.71; APP: 9.7 ± 0.3; n = 50 ultrathin sections from 9 animals, P < 0.001; p25: 10.2 ± 0.3, n = 50 ultrathin sections from 9 animals, P < 0.001; Mann–Whitney Rank Sum tests compared with Ctrl: 12.5 ± 0.4, n = 50 ultrathin sections from 9 animals), and 7-day (CFP: 12.5 ± 0.3, n = 50 ultrathin sections from 8 animals, P = 0.89; APP: 8.1 ± 0.3; n = 50 ultrathin sections from 8 animals, P < 0.001; p25: 7.2 ± 0.3, n = 50 ultrathin sections from 8 animals, P < 0.001; Mann–Whitney Rank Sum tests compared with Ctrl: 12.7 ± 0.4, n = 50 from 8 animals) in vivo overexpression. (Right) Relative PSD length in control nonexpressing CA1 neurons, CA1 neurons overexpressing CFP, p25-RFP, or APP-GFP at different expression time. Values for the average PSD length of each group after 2-day (CFP: 228.3 ± 3.3 nm, n = 619 synapses from 8 animals, P = 0.81; APP: 225.7 ± 4.0 nm, n = 526 synapses from 8 animals, P = 0.80; p25: 238.4 ± 3.9 nm, n = 545 synapses from 8 animals, P < 0.01; Mann–Whitney Rank Sum tests compared with Ctrl: 225.2 ± 3.5 nm, n = 625 from 8 animals), 4-day (CFP: 224.2 ± 2.9 nm, n = 616 synapses from 8 animals, P = 0.85; APP: 230.4 ± 2.9 nm, n = 484 synapses from 9 animals, P = 0.07; p25: 272.7 ± 3.0 nm, n = 510 synapses from 9 animals, P < 0.001; Mann–Whitney Rank Sum tests compared with Ctrl: 222.9 ± 2.7 nm, n = 626 synapses from 9 animals), and 7-day (CFP: 226.7 ± 3.7 nm, n = 623 synapses from 8 animals, P = 0.90; APP: 227.9 ± 4.7 nm, n = 407 synapses from 8 animals, P = 0.73; p25: 244.9 ± 5.0 nm, n = 361 synapses from 8 animals, P < 0.005; Mann–Whitney Rank Sum tests compared with Ctrl: 229.4 ± 4.3 nm, n = 595 from 8 animals) in vivo overexpression. The relative values and standard errors were normalized to average mEPSC frequency, amplitude, synapse density, and PSD length from control cells. Note the significantly more reductions in mEPSC frequency and synapse density in p25-expressing CA1 neurons compared with APP-expressing neurons after 7-day overexpression. Asterisks indicate P < 0.05. (H) A schematic model describes physiological and pathological Cdk5 signaling.