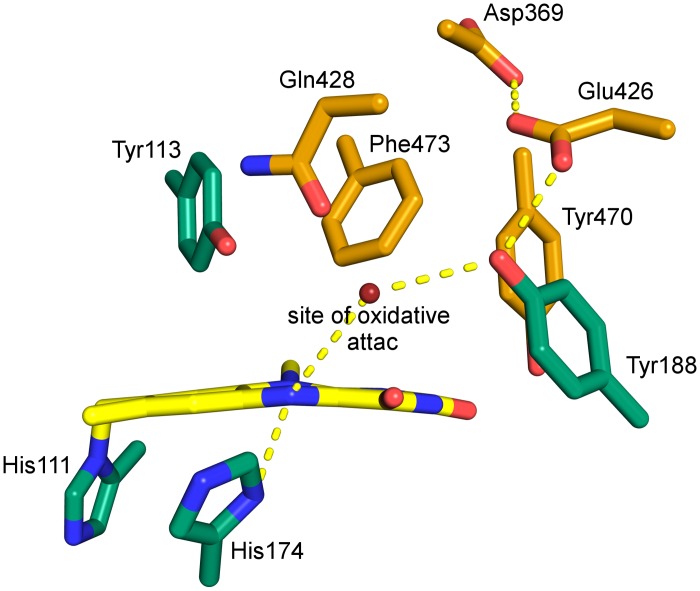
Fig 8. Model of the active site of AtBBE-like 28 indicating the putative site of oxidative. attack (red sphere).
Interactions are represented by dashed yellow lines. One of the unique features of the type IIa active site is the nature and position of the catalytic base, i.e. Tyr188 that interacts with Glu426 and a monocovalent attachment of the cofactor. In the active site type IIb, nature and position of the catalytic base are conserved (compare Fig 7 1.1 and 1.2) but a bicovalent attachment of the cofactor is to be expected for this active site type. Interestingly the change from a bicovalent to a monocovalent binding mode goes along with the variation of two amino acids of the oxygen reactivity motif. These are the amino acids at position 174 (i.e. cysteine to histidine exchange), responsible for the formation of the covalent bond to C(6) of the isoalloxazine ring and 182 (i.e. histidine to glutamine exchange) (Fig 7). This is perfectly in line with findings reported by Kopacz et al. [33] on 6-hydroxy-D-nicotine oxidase, a VAO-type flavoprotein with a monocovalent linkage of a histidine to the 8α-position of the isoalloxazine ring. In this enzyme, bicovalent attachment was achieved by introduction of a cysteine and histidine residue corresponding to position 174 and 182, respectively, indicating that the histidine residue plays a crucial role in the formation of the sulfur-carbon bond [33].