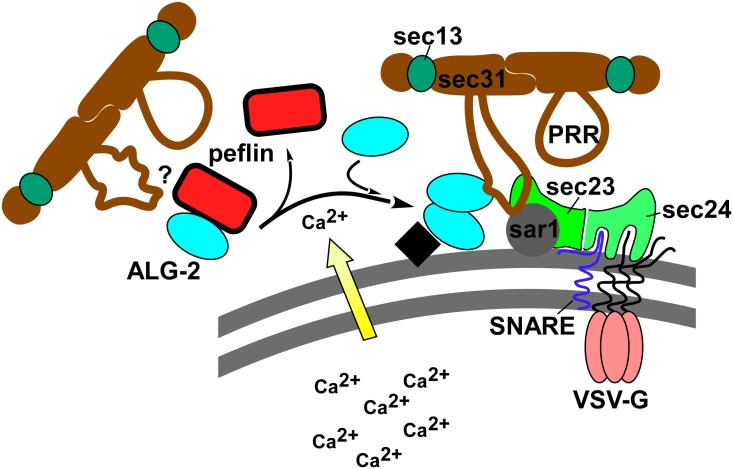
Fig 7. Model for peflin regulation of ER-to-Golgi transport.
Peflin exists as a heterodimer with its binding partner ALG-2. In the presence of calcium peflin dissociates, allowing ALG-2 to homodimerize and undergo Ca2+-dependent binding to the proline rich region (PRR) of sec31A. ALG-2 binding to the PRR activates the PRR for interactions with inner shell components sar1 and sec23, which stabilizes the coat assembly on the membrane. These events may increase ER-to-Golgi transport. However, we cannot eliminate the possibility that peflin may also or instead regulate transport through unknown interactions. It is also not known whether the peflin-ALG-2 heterodimer binds the PRR region and affects its activity ("?" to left of heterodimer). The black square represents a hypothetical docking site for ALG-2 at the ERES.