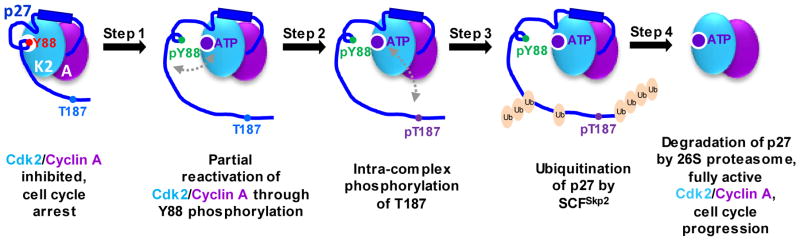
Figure 1.
p27 as a signaling conduit. Tyrosine phosphorylation-dependent partial unfolding of p27 triggers signal propagation through the length of the protein and regulates its degradation. Step 1 involves phosphorylation of Y88 of p27 that is bound to Cdk/cyclin complexes [Cdk2 (K2)/cyclin A (A) here] by non-receptor tyrosine kinases such as BCR-ABL, Src, Lyn, and Jak2, which ejects Y88 from the ATP binding pocket of Cdk2 and restores partial kinase activity. Following Step 1, Step 2 involves phosphorylation of T187 within the flexible C-terminal domain of p27 by partially active Cdk2 through a pseudo uni-molecular mechanism (indicated by gray arrow). Phosphorylation of T187 creates a phosphodegron signal for ubiquitination of Lysine residues within the p27 C-terminus by the E3 ligase, SCFSkp2, during Step 3. Finally, during Step 4, ubiquitinated p27 is selectively degraded by the 26S proteasome, leading to the release of fully active Cdk2/cyclin A, which drives progression into S phase of the cell division cycle.