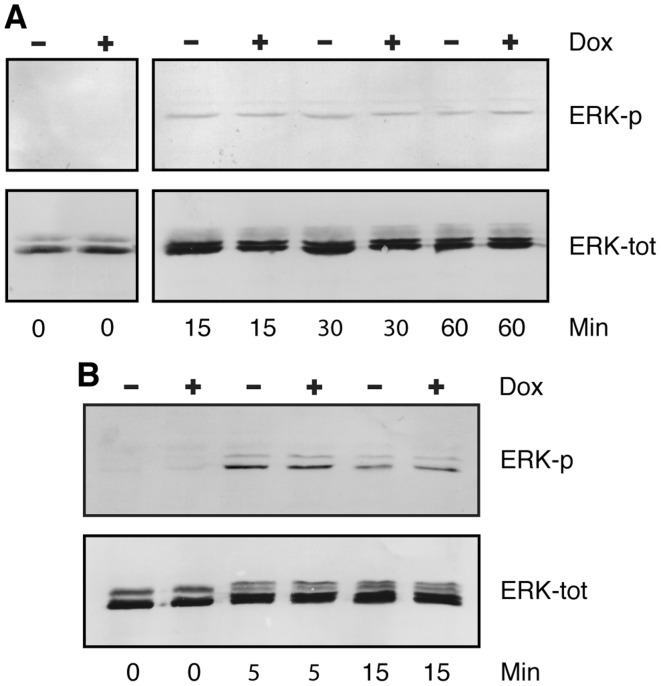
Figure 4.
Effect of fibroblast growth factor receptor-like protein 1 (FGFRL1) on ERK phosphorylation. FGFRL1 inducible HEK-TetOn cells were cultivated in multi-well plates, starved overnight in medium lacking fetal bovine serum and then stimulated for 0–60 min as indicated with human FGF2 (15 ng/ml). Cells were lysed with hot SDS sample buffer. Cellular proteins were resolved on polyacrylamide gels and processed for western blotting with antibodies against phosphorylated ERK1/2 (ERK-p). The blots were stripped and reprobed with antibodies against total ERK (ERK-tot). An experiment conducted with clone K24F is depicted in panel A. Panel B shows an experiment with clone K17F. However in this case, the cells had been transfected - prior to starvation and FGF2 stimulation - with a full-length clone for human FGFR1 to increase signaling. ERK1/2 phosphorylation was extremely low before stimulation, but clearly visible after stimulation with FGF2. No difference in ERK phosphorylation was observed between doxycycline-induced and uninduced cells.