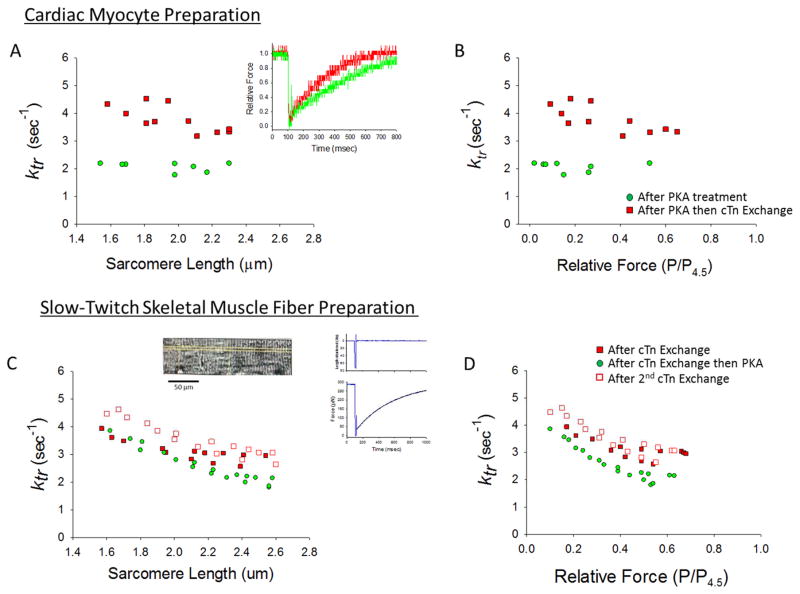
Figure 3. Effects of unphosphorylated RcTn exchange on rate constant of force development (ktr) in skinned rat cardiac myocytes and slow-twitch skeletal muscle fibers.
(A & B) A skinned cardiac myocyte preparation was first treated with PKA and the rate of force development was measured over a range of sarcomere lengths (green circles). Then the skinned myocyte preparation underwent exchange with unphosphorylated RcTn, which increased ktr at all sarcomere lengths (A) and relative force levels (B). Inset in A shows force redevelopment traces in a myocyte preparation with unphosphorylated cTnI and PKA induced phosphorylated cTnI. (This experiment was performed on 4 myocyte preparations). (C & D) In a skinned slow-twitch skeletal muscle fiber, the sarcomere length (C) and relative force (D) dependence of ktr was sequentially measured after an initial 12 hours of exchange with unphosphorylated RcTn (filled red squares), then after the fiber was treated with PKA (this decreased ktr) (green circles), and then after an additional 12 hours of RcTn exchange (which returned ktr to higher values) (open red squares). Inset shows an image of a slow-twitch skeletal muscle fiber and a force redevelopment trace after a slack-restretch maneuver. (This experiment was performed on 4 slow-twitch skeletal muscle fibers).